Download Download & share this Knowledge card in your network [Free Download]
Table of Contents
- Introduction
- History of Cerebral Folate Deficiency Syndrome
- Five mechanisms that can contribute to CFDS
- Cerebral Folate Deficiency Syndrome, Age and Folate Receptor Alpha Autoantibodies
- Diet, Folate Receptor Alpha Autoantibodies and CFDS
- Treatment
- Did You Know About Folate Receptor Autoantibodies (FRAAs) and Brain Development?
Introduction
Cerebral folate deficiency syndrome (CFDS) is defined as any neuropsychiatric or developmental disorder characterized by decreased CSF folate levels in the presence of normal folate status outside the nervous system. The primary cause of CFDS is identified as the presence of serum folate receptor alpha (FRα) autoantibodies impairing folate transport across the choroid plexus to the brain whereas, in a minority of cases, mitochondrial disorders, inborn errors of metabolism and loss of function mutations of the Folate Receptor alpha gene are identified. Early detection and diagnosis of CFDS and prompt intervention are important for a good prognosis.
Download Download & share this Knowledge card in your network [Free Download]
History of Cerebral Folate Deficiency Syndrome
In 2004, the term Cerebral Folate Deficiency (CFD) was created to define any neuropsychiatric or neurodevelopmental disorder associated with low MTHF (methyl tetrahydrofolate) concentration in CSF (Cerebrospinal Fluid) in the presence of normal folate, vitamin B12 and homocysteine outside the nervous system.
Folate, otherwise known as Vitamin B9, is a critical nutrient. The physiological function of its most reduced form (methyl tetrahydrofolate) is necessary for many functions including the de novo synthesis of purines and thymidine, methylation of DNA, the conversion of homocysteine to methionine, and the formation of the active methyl-group donor S-adenosylmethionine (SAM), which is used for the transfer of methyl-groups. SAM is used in more than 100 chemical reactions among which are the methylation of DNA, fatty acids, phospholipids, polysaccharides, and proteins. Adequate folate is not only necessary for DNA synthesis and replication, cell division, growth, and survival, but also for normal embryonic growth, as well as development and maturation of the nervous system.
Functional transport mechanisms across the intestinal, placental, and blood-brain barriers are crucial to achieve adequate folate within organs and especially into the CNS. For passage across the blood–CSF barrier, plasma 5MTHF is bound to the Folate Receptor Alpha. The choroid plexus is the main site of active folate transport to the CNS, as the Folate Receptor Alpha possesses a high affinity for folate. Folate transport by Folate Receptor Alpha across the choroid plexus is an active energy dependent transport, leading to a two-fold higher concentration.
However, when folate transport is impeded into the Brain and the CSF, a condition now known as Cerebral Folate Deficiency Syndrome (CFDS) arises. Simply put, CFDS occurs due to the impaired transport of folates across the blood-brain barrier and cerebrospinal fluid.
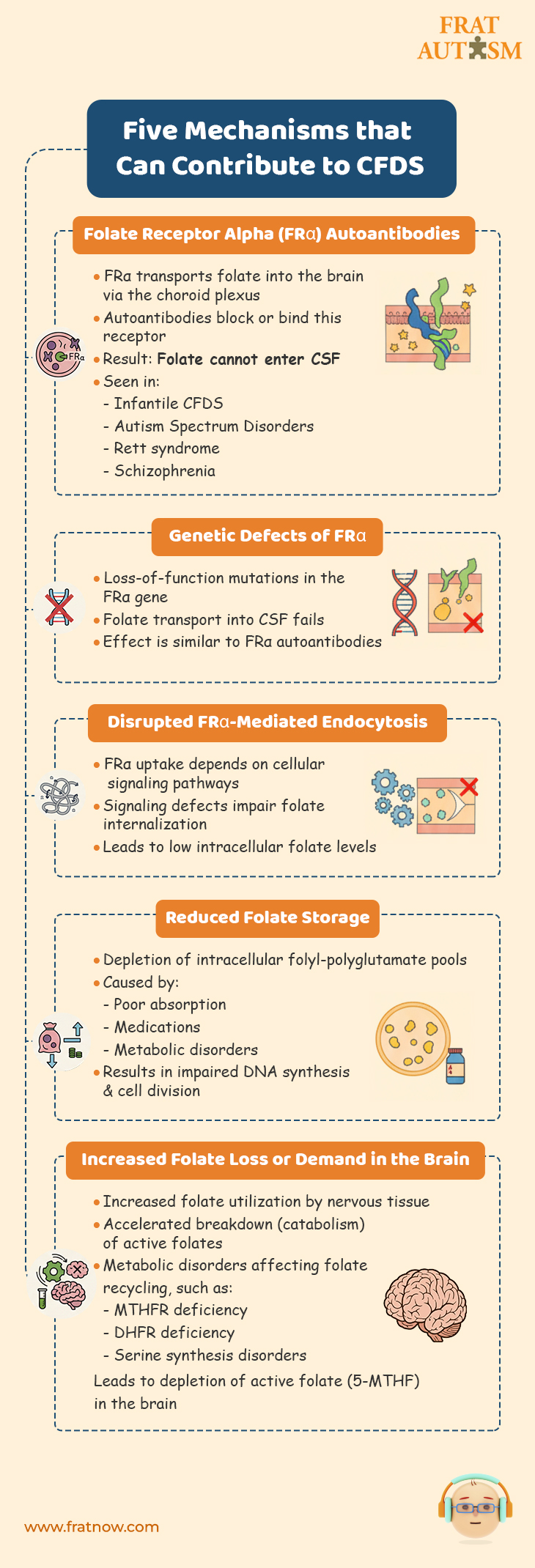
Five mechanisms that can contribute to CFDS
- Reduced transport of folates across the blood–brain barrier into the CSF caused by :
- Folate Receptor Alpha Autoantibodies :
Folate Receptor alpha is the primary folate receptor at the choroid plexus that transports folate in the CFS. It has been found that a large number of cases of CFDS involve the presence of folate receptor autoantibodies (blocking and binding types). These folate receptors autoantibodies make the folate receptor alpha dysfunctional, thereby causing a form of cerebral folate deficiency. In other words, not enough folate can make it in the CSF, which has a critical need for it. Folate Receptor Alpha autoantibodies have been found in infantile-onset CFD, spastic-ataxic CFD, dystonia CFD, Autism Spectrum Disorders, Rett syndrome, Aicardi-Goutières, and schizophrenia. - Genetic defects leading to loss of function of the FRα :
Similar to how Folate Receptor Alpha autoantibodies make the folate receptor dysfunction, gene defects will have similar consequences with regards to folate transport into the CFS. - Signal transduction disorders affecting the regulation of FRα-mediated endocytosis:
FRα is a cell surface receptor that plays a crucial role in the internalization of folate. The regulation of FRα-mediated endocytosis is tightly controlled by signal transduction pathways that transmit signals from the cell surface to the intracellular machinery involved in endocytosis.
Disruptions or abnormalities in the cellular signaling pathways that regulate the process of endocytosis mediated by the folate receptor alpha (FRα) can wreak havoc with the functionality of the folate receptor alpha resulting in impaired uptake of folate by cells, decreased intracellular folate levels and potential folate deficiency.
- Folate Receptor Alpha Autoantibodies :
- Reduced folate storage and release from the intracellular folyl-polyglutamate pool.
Decreased folate storage due to depletion of the intracellular folyl-polyglutamate pool refers to a condition in which the body’s ability to store folate is reduced. Intracellular folyl-polyglutamates are the active forms of folate found inside cells. These forms of folate are involved in many cellular processes and are crucial for maintaining an adequate supply of folate for cellular functions. When the intracellular folyl-polyglutamate pool is depleted, it means that the levels of active folate forms within the cells are reduced. This depletion can be caused by factors such as inadequate dietary intake of folate, impaired absorption of folate from the diet, certain medications, or underlying medical conditions that interfere with folate metabolism. As a consequence of decreased folate storage, the body may not have enough folate to support essential cellular processes. This can lead to impaired DNA synthesis, and problems with cell division. - Increased utilization and consumption of reduced folates within the nervous system leading to depletion of the folate pool.
Increased utilization and consumption of reduced folates within the nervous system means that the nervous tissue requires higher amounts of folate for its normal functioning. This can be due to increased metabolic demands or underlying conditions that require more folate for proper neurological processes.
As a result, the nervous system utilizes folate at a higher rate, leading to a depletion of the folate pool in the body. - Increased catabolism of reduced folates within the nervous system.
The process of breaking down or degrading reduced folates, which are the active forms of folate, is accelerated specifically within the nervous system.
Increased catabolism of reduced folates within the nervous system means that the breakdown or degradation of these active folate forms is happening at a higher rate. This can be due to various factors, including genetic abnormalities, enzyme deficiencies, or metabolic dysregulation specific to the nervous system. As a result of increased catabolism, the available levels of active folates, such as 5-MTHF, decrease within the nervous system. This can lead to a deficiency or insufficiency of folate for essential cellular processes in the nervous system, including DNA synthesis, methylation reactions, and neurotransmitter synthesis. - Metabolic conditions affecting folate metabolism within the nervous system.
Normal processes involved in folate uptake, utilization, or recycling are disrupted or impaired and these conditions can arise due to genetic mutations, enzyme deficiencies, or other metabolic dysfunctions. Such metabolic conditions can result in altered folate levels or impaired folate utilization. These may include :- Methylenetetrahydrofolate reductase deficiency
- Serine hydroxymethyltransferase deficiency
- Dihydrofolate reductase deficiency
- Dihydropteridine reductase deficiency
- Depletion of methyl-donor pool glycine, serine, and histidine
- Disorders of serine synthesis
- Glutamate Formiminotransferase deficiency
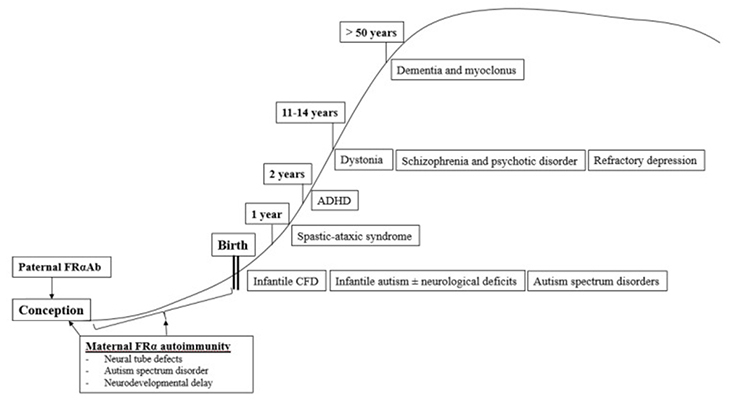
Cerebral Folate Deficiency Syndrome, Age and Folate Receptor Alpha Autoantibodies
The first described clinical syndrome of CFDS, called infantile-onset CFD, resembles hereditary folate malabsorption with respect to neurological features, but with less severe clinical profiles of hematological, intestinal, or immunological abnormalities as seen in hereditary folate malabsorption.
Infantile CFDS manifests as sleeping problems, unrest, and agitation, decreased growth, psychomotor retardation with hypotonia and ataxia, dyskinesias, and/or epilepsy between 4–6 months of age. In untreated patients, visual loss developed from the age of three and bilateral hearing loss from the age of six years. Additionally, Infantile CFD syndrome is characterized by the absence of basal ganglia calcifications, in contrast to hereditary folate malabsorption. MRI brain scans show delayed myelination, demyelinated subcortical and periventricular areas, as well as cerebral and cerebellar atrophy in half of untreated CFD cases. The results of all laboratory tests were normal, including hematology, folate, B12, homocysteine, immunoglobulins, amino acids, and tests for inborn errors of metabolism.
The most prevalent cause of infantile-onset CFDS appears to be serum folate receptor alpha autoantibodies directed against the choroid plexus, which impairs MTHF binding across the choroid plexus and passage to the spinal fluid compartment. A number of children with infantile-onset CFD syndrome develop autism. It is important to suspect infantile CFD as in these cases early diagnosis and treatment with folinic acid treatment can prevent further neurologic deterioration. In fact, some children treated before the age of two years can recover completely.
In comparison to infantile-onset CFDS, children with low-IQ autism and typical neurological features also had low CSF MTHF levels and similar serum FRα autoantibody titers. In further studies, FR autoantibodies were absent in parents of children with infantile-onset CFDS, while both parents or either parent had autoantibodies in children with low-IQ autism and features of CFDS. Since both sperm and oocytes depend on folate receptor alpha to transport folate, folate receptor autoimmunity in either or both parents might be associated with development of autism and or autistic symptoms in their offspring. An insufficient amount of folate in male sperm or oocytes will affect DNA and cause abnormal pathways affecting folate-dependent metabolism, epigenetic gene activation/silencing, and neurodevelopment. Additionally, the presence of folate receptor autoantibodies in mothers will further impair normal embryonic and fetal development, with a predisposition to neural tube defects (NTD) and infantile autism.
In a study of children with infantile autism, without neurological deficits, CSF MTHF levels weren’t as low as in infantile CFD or low-IQ autism with neurological deficits, but still low or within the low normal reference range with a statistical difference compared to healthy controls who had normal CSF folate levels. Interestingly, their folate receptor alpha autoantibody titers were in the same range as those for children with infantile CFD or low-IQ autism with neurologic abnormalities. In contrast to healthy age-matched controls of children and their parents, a significant proportion of parents of infantile autism children had folate receptor autoimmunity. This leads to the conclusion that in children with infantile autism, folate receptor alpha autoimmunity can be acquired after birth by the child or can be present in one or both parents.
It has also been determined that the occurrence of postnatal folate receptor alpha autoantibodies in children between 1 and 2 years results in a spastic-ataxic CFD syndrome associated with learning deficits.
Between 2 and 5 years, FRα autoimmunity was observed in a low proportion of children suffering from attention deficit hyperkinetic disorder (ADHD), learning deficits and other behavioral abnormalities.
In many patients with CFD syndromes that present in childhood, adolescence, and adulthood, folate receptor alpha autoimmunity is present as well.
During adolescence or adulthood, FRα autoimmunity can predispose to severe psychotic episodes and refractory schizophrenia. Other psychiatric conditions associated with FRα autoimmunity predisposing to CFD manifest as severe treatment-resistant major depression. Folinic acid therapy in these cases shows efficacy.
In summary, the occurrence of a specific neurologic or psychiatric condition associated with CFDS due to FRα autoimmunity depends on the age of onset when these FRα autoantibodies develop and, on the presence, or absence of parental FRα autoimmunity, particularly among families with children suffering from autism spectrum disorder.
Diet, Folate Receptor Alpha Autoantibodies and CFDS
Studies indicate that the human FRα protein antigen shows around 90% amino acid sequence homology to FRα antigen present in all animal-derived milk and milk products. Among genetically susceptible patients, the ingestion of cow´s milk after birth predisposes the intestinal immune system to produce antibodies against the soluble FRα antigen present in cow’s milk which can enter the circulation and cross-react with the FRα antigen attached to the choroid plexus, thyroid, and gonads. This connection may explain the inception of folate receptor alpha autoantibodies.
Treatment
After diagnosis of CFD syndrome caused by FRα autoimmunity, treatment is generally given in the use of pharmaceutical doses of folinic acid or levo-5-methyl-tetrahydrofolate. Another therapeutic option, which can be combined with folinic acid treatment, is the introduction of a strict animal-milk-free diet. This will eliminate exposure to soluble FR from animal-derived milk products.
One of the most important aspects in the clinical management of infantile-onset CFDS, autism with neurologic deficits or infantile autism is to diagnose these conditions as early as possible since early treatment with folinic acid for FRα autoimmunity has shown to considerably improve outcomes.
Age-dependent classification of CFDS.
For information on autism monitoring, screening and testing please read our blog.