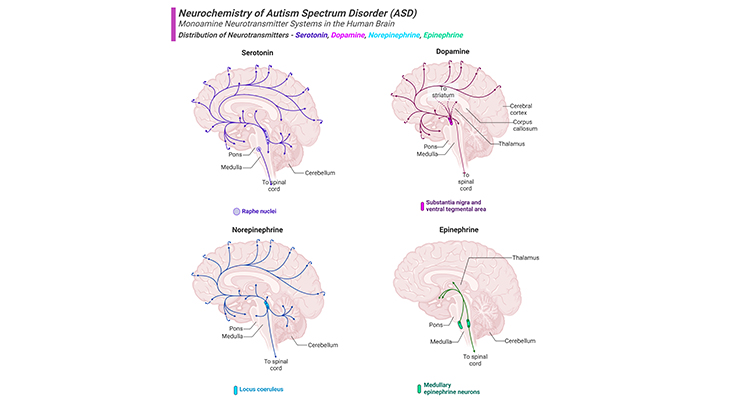
Figure 1. Distribution of Biogenic Amine Neurotransmitters in the Human Brain.
Current knowledge dictates that neurotransmitter system dysfunction could be the cause of autism spectrum disorder (ASD), by affecting neuronal migration, differentiation, synaptogenesis, and eventually developmental process of the brain. Major projection pathways of the biogenic amine neurotransmitter systems in the human brain. Shown are the projections of the serotonin, dopamine, norepinephrine, and epinephrine systems using the neurotransmitters. The views are midsagittal sections through the human brain, depicting the medial surface of the right hemisphere; the front pole is at left. In each image, the primary source of the biogenic amine is bold type and colored accordingly.
Introduction
Neurotransmitters, also called chemical transmitters or chemical messenger, any of a group of chemical agents released by neurons to stimulate or inhibit neighboring neurons or muscle or gland cells, thus impulse to be propagated from one cell to the next throughout the nervous system [1]. Neurotransmitters are chemical messengers by which neurons communicate with each other, and play crucial roles in normal development of brain, memory, motor activity, and behavior regulation. Current knowledge dictates that neurotransmitter system dysfunction could be the cause of autism spectrum disorder (ASD), by affecting neuronal migration, differentiation, synaptogenesis, and eventually developmental process of the brain. In this context, several neurotransmitter systems have been investigated and dysfunction of these systems has been demonstrated to be responsible for the pathophysiology of ASD. In the literature, neurotransmitters that are most commonly associated with the pathogenesis of ASD are serotonergic, glutamatergic, GABAergic, and dopaminergic systems (Figure 1).
I. What is known
Even though there is no general consensus concerning the exact nature of neurochemical brain abnormalities in individuals with ASD. However, there is ample evidence that several of the major neurochemicals that are classified as neurotransmitters, neuromodulators, and neurotrophins, are affected one way or another. Here we have summarized evidence pertaining to each of these neurochemical substances in ASD, in an order that reveals the established significance from best to least well established in a sequential manner [2]. (cf. previous blog entitled as ‘The Neurotypical Brain versus the Autistic Brain: Neurotypical Brain Chemistry)
(i) Serotonin: Serotonin (aka: 5-hydroxytrptamine or 5-HT) is a monoamine chemical messenger derived from the amino acid tryptophan that plays significant roles in many organ systems, including the cardiovascular, gastrointestinal, and central nervous systems. Even though most of the body’s serotonin is located within platelets and enterochromaffin cells, its function as a neurotransmitter in the central nervous system (CNS) is particularly prominent. Serotonin in the brain is most produced by neurons located in the raphe nucleus in the brainstem, and it regulates several brain functions, including mood, sleep, and satiety, via widespread projections of these neurons towards multiple brain areas, including the cerebral cortex, limbic system, midbrain, and hind brain (Figure 2).
Serotonin is transmitted from one neuron to another neuron by a serotonin transporter (SERT or 5-HTT) substance. 5-HTT is also known as the sodium-dependent serotonin transporter and solute carrier family 6 member 4, is a protein that in human is encoded by the SLC6A4 gene.
Neurons communicate by using chemical messengers like serotonin between cells. The transporter protein, by recycling serotonin, regulates its concentration in a gap or synapse, and thus its effects on a receiving neuron’s receptors. SERT/5-HTT is a type of monoamine transporter protein that transports the neurotransmitter serotonin from the synaptic cleft back to the presynaptic neuron, in a process known as serotonin reuptake; thus plays an important role in the pathophysiology of several psychiatric and/or neuro-psychiatric disorders, including ASD.
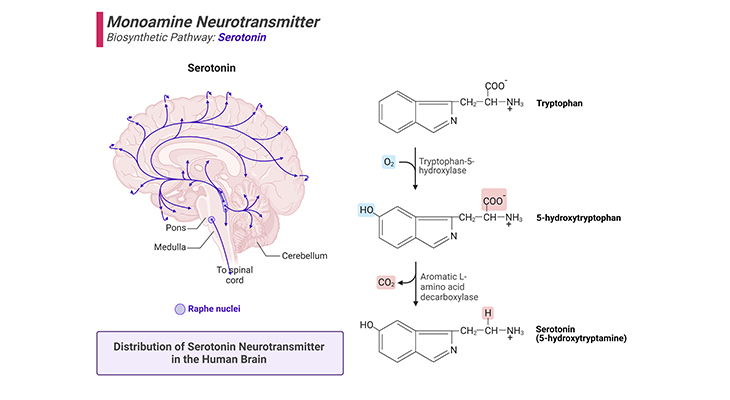
Figure 2. Biosynthetic Pathway and Distribution of Serotonin Neurotransmitter in the Human Brain.
(a) Serotonin is derived from the amino acid tryptophan by a two-step process that requires the enzymes tryptophan-5-hydroxylase and a decarboxylase (right panel). (b) Major projection pathway of the monoamine neurotransmitter system in the human brain. Shown are the projections of the serotonin system using the neurotransmitter (left panel). The views are midsagittal sections through the human brain, depicting the medial surface of the right hemisphere; the front pole is at left. In each image, the primary source of the biogenic amine is bold type and colored accordingly.
Abnormalities of serotoninergic system or its expression in the human brain could therefore arise in any one of the following three ways:
- Hyper- or hypo-production of serotonin (5-HT) itself.
- A problem with the serotonin transporter substance, 5-HTT/SERT.
- Abnormalities in the provision and action of serotoninergic receptor cells.
The potential effects of serotonin-related abnormalities in ASD are wide-ranging, given the fact that serotonin acts not only as a neurotransmitter, but also as a neuromodulator, and prenatally, as a neurotrophins. Several scientific reports have evidently shown that all three abnormalities occur more often in subjects with ASD than in neurotypical (NT) individuals. However, none of these three types of abnormalities co-occurs in every autistic subject, or even for that matter in a majority of subjects with ASD [3].
(ii) Glutamate and GABA: As a rule, glutamate is the most abundant excitatory neurotransmitter, and GABA (gamma-aminobutyric acid) the most abundant inhibitory neurotransmitter, operating throughout the brain. The normal expression of both these chemical substances, i.e., production, transportation, and reception, is essential to maintain a balance between hyper- (too much) and hypo- (too little) neural activity. Thus, any abnormalities associated with either of these neurotransmitters may potentially perturb this balance in one or the other direction (Figure 3; Figure 4).
At the level of brain, the theory that ASD might result from an imbalance between the major excitatory and inhibitory neurotransmitters, glutamate and GABA was initially pronounced. The subsequently named ‘EI theory (Emotional Intelligence)’ elicited considerable interest among autism researcher. Thereafter, in numerous studies, reduced levels of GABA have now been demonstrated in the brains of individuals with ASD [4]. Moreover, there is also some evidence to suggest that a surplus of glutamate is detected in the brains of individuals with ASD. Most significantly, even if the levels of glutamate expression are within normal limits in ASD, decreased inhibitory control associated with reduced GABA expression by itself lead to higher-than-normal levels of excitation, and ‘noise’ in the neural transmission system.
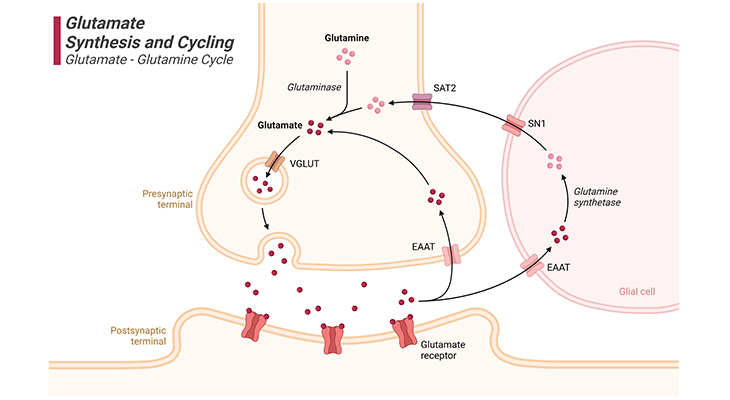
Figure 3. Glutamate Synthesis and Cycling.
Glutamate synthesis and cycling between neurons and glia, the so-called ‘glutamate – glutamine cycle.’ Glutamate is the major excitatory neurotransmitter in the brain. Glutamate is a nonessential amino acid that does not cross the blood-brain barrier (BBB) and must be synthesized from local precursors. Glutamine is the most prevalent precursor in synaptic terminals for glutamate synthesis. Glutamine is released by glial cells and, once within the presynaptic terminal of the neuron, it is metabolized to glutamate by the mitochondrial enzyme glutaminase. Glutamate can also be synthesized by the process of transamination of 2-oxoglutarate, an intermediate of the tricarboxylic acid (TCA) cycle. Thus, some of the glucose metabolized by neurons can also be utilized for glutamate synthesis. [VGLUT – vesicular glutamate transporter; EAAT – excitatory amino acid transporter; SN1 – system N1 transporter (glutamine transporter); SAT2 – system A transporter]
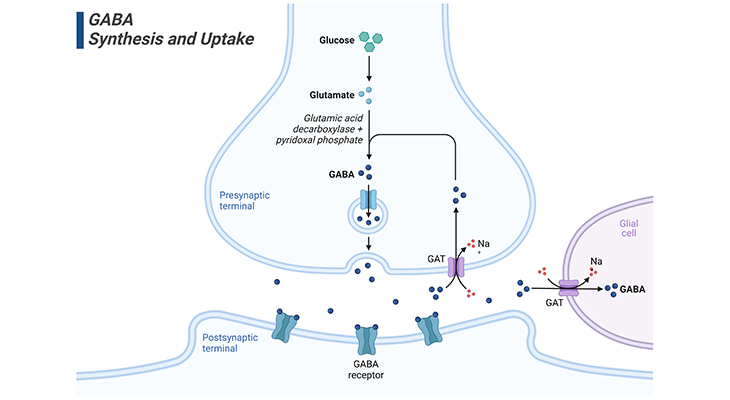
Figure 4. GABA Synthesis, Release, and Uptake.
GABA is the major inhibitory neurotransmitter in the brain. Glucose is the predominant precursor for GABA synthesis. Initially, glucose is metabolized to glutamate by the tricarboxylic acid (TCA) cycle enzymes, although glutamine and pyruvate can also act as precursor substances. GABAergic neurons express glutamic acid decarboxylase (GAD), which is found almost uniquely in these neurons. GAD catalyzes the conversion of glutamate to GABA. GAD requires pyridoxal phosphate as a cofactor for optimal activity. Since pyridoxal phosphate is derived from vitamin B6, a B6 deficiency can lead to reduced GABA synthesis. ‘The significance of this become obvious after a disastrous series of infant deaths, linked to the omission of vitamin B6 from infant formula.’ The absence of B6 resulted in a substantial reduction in GABA content of the brain, and the ensuing loss of synaptic inhibition caused seizures in newborns that in some cases were fatal. More recently, seizures due to B6 deficiency have been reported in adults. [GABA – gamma amino butyric acid; GABA receptors – gamma amino butyric receptors; GAT – GABA transporter type 1, 2, or 3]
(iii) Dopamine: As in the case of serotonin, there are clearly demonstrated abnormalities, conceivably of various kinds, in the dopaminergic system in individuals with ASD [5]. Besides, it has long been suggested that dysfunctioning of the dopaminergic system contributes to motor abnormalities in ASD (Figure 5).
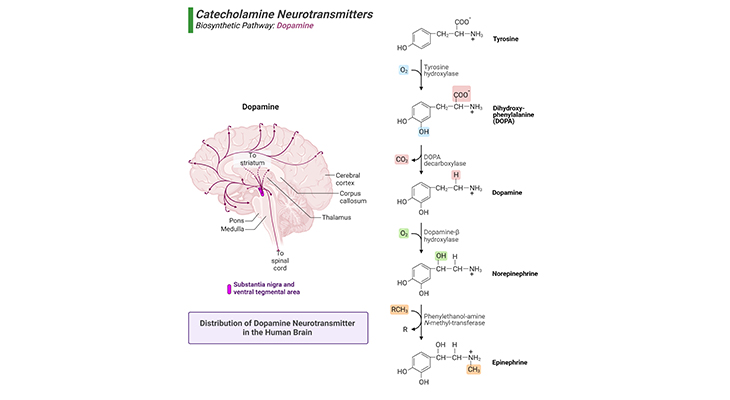
Figure 5. Biosynthetic Pathway and Distribution of Dopamine Neurotransmitter in the Human Brain.
(a) Dopamine is synthesized from the amino acids, phenylalanine or tyrosine via sequential reactions catalyzed primarily by phenylalanine hydroxylase, tyrosine hydroxylase, and DOPA decarboxylase (right panel). (b) Major projection pathway of the biogenic amine neurotransmitter system in the human brain. Shown are the projections of the dopamine system using the neurotransmitter (left panel). The views are midsagittal sections through the human brain, depicting the medial surface of the right hemisphere; the front pole is at left. In each image, the primary source of the biogenic amine is bold type and colored accordingly.
In recent years, abnormalities of dopamine production, transmission, and reception have been associated with the following:
- Emotion dysregulation
- Anomalies of attention
- Executive dysfunction
- Repetitive behavior
- Motor abnormalities
(iv) Acetylcholine: Unlike the serotonergic and dopaminergic systems, the cholinergic system was not vigorously studied in individual with ASD until the beginning of the current century. A post-mortem study, nevertheless, showed reduction in the numbers of acetylcholine receptors (the so-called ionotropic nicotinic receptors, nAChRs), in the parietal lobes of individuals with ASD [6]. Since then, it has been determined that the reduced numbers of nicotinic receptor cells are found to be widespread in the brains of autistic individuals; with possible implications for understanding disabilities of attention, memory and learning, as well as possibly the imbalance between excitatory and inhibitory brain activity in ASD. In this context, the case for developing treatments essentially targeting nicotinic receptor cells has been widely discussed.
(v) Oxytocin: The role of oxytocin in sexual behaviors and childbirth was known nearly a century ago. Oxytocin is a naturally occurring nine-peptide amino acid, synthesized in the hypothalamus and stored and released by the posterior pituitary. Oxytocin is important in a broad range of behaviors, including bonding, nurturing, and social recognition. Through these behaviors, along with others, oxytocin is thought to facilitate the development of attachment and social skills. For example, in humans, parental oxytocin levels have been associated with parental touch and engagement, thus creating a ‘parent-infant dyad’ through which bonding and learning occur. Considering its role in social behavior, the potential of oxytocin as a therapeutic agent for social difficulties in ASD is a focus of current research (Figure 6).
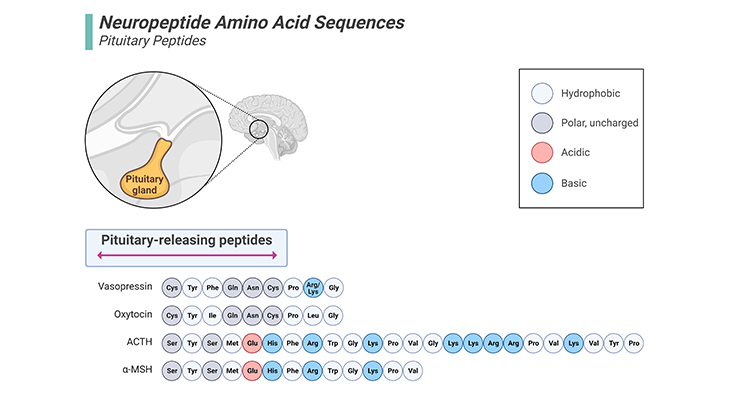
Figure 6. Neuropeptide Amino Acid Sequences – Pituitary Peptides.
Oxytocin is a naturally occurring nine-peptide amino acid that is synthesized in the hypothalamus and stored and released by the posterior pituitary. Oxytocin is important in a broad range of behaviors, including bonding, nurturing, and social recognition. Vasopressin is structurally similar to oxytocin. Vasopressin is a nine-amino acid peptide hormone synthesized by hippocampal magnocellular and parvocellular neurons. It is synthesized as pre-pro-vasopressin in the neuron cell bodies and is cleaved into vasopressin prior to reaching the posterior pituitary, where it is then released. Vasopressin is involved in multiple systemic functions, including maintenance of cardiovascular homeostasis, increased water retention, and regulation of the body’s response to stress. [ACTH – adreno corticotropic hormone; α-MSH – alpha-melanocyte-stimulating hormone]
- Oxytocin, a naturally occurring peptide, is key for social perception, learning, and mediating pair bonding in animals and humans.
- Three key genetic pathways modulate oxytocin expression, receptors, and release. These have been linked to individual differences in social cognition, behavior, and risk for ASD.
- Nasal administration of oxytocin has been associated with increased social engagement and aptitude along with alterations in functional brain activity.
- There is evidence for oxytocin action across social communication and restricted and repetitive behavior domains, in reductions in anxiety, and in alterations in sensory sensitivity.
- Oxytocin interventions studies have had inconclusive findings, although particular promise has been found for pediatric ASD.
Findings from a spate of research studies are, however, quite mixed [7]. So, the jury is still out as to the role or reduced oxytocin production/transportation/reception as a contributory case of ASD; also, as to the usefulness of oxytocin-based treatments.
(vi) Vasopressin: Vasopressin is a nine-amino acid peptide hormone synthesized by hippocampal magnocellular and parvocellular neurons. It is synthesized as pre-pro-vasopressin in the neuron cell bodies and is cleaved into vasopressin prior to reaching the posterior pituitary, where it is then released. It is involved in multiple systemic functions, including maintenance of cardiovascular homeostasis, increased water retention, and regulation of the body’s response to stress (Figure 6).
Besides, vasopressin, through neuronal projections in the brain, is considered to be involved in various social and cognitive functions, including anxiety, fear, and aggression. Vasopressin is structurally similar to oxytocin; preclinical and clinical evidence suggests that these hormones work together to influence social behaviors, including love, sexual behavior, and parental behavior. Using animal studies, it has been shown that the hormone vasopressin has some role in the regulation of social behavior. On these grounds, it has been postulated to play a role in causing ASD, and studies of oxytocin and vasopressin are frequently undertaken together [8].
- Vasopressin has been implicated in ASD through studies investigating its concentrations, genetic linkage, and receptor expression. However, evidence across these studies is varied and often contradictory.
- Preclinical evidence suggests that vasopressin influences social communication behavior to a certain extent.
- Preliminary clinical data investigating V1AR (vasopressin receptor 1A or arginine vasopressin receptor 1A, AVPR1A) antagonism and vasopressin administration also indicated that vasopressin has a role in social communication in individuals with and without ASD.
(vii) Brain-derived neurotrophic factor (BDNF) and Reelin: BDNF and Reelin, in humans, are encoded by the BDNF and RELN genes, respectively [9 -10].
BDNF is one of the neurotropic factors that supports differentiation, maturation, and survival of neurons in the nervous system. BDNF exhibits neuroprotective effects under adverse conditions, for example, glutamatergic stimulation, cerebral ischemia, hypoglycemia, and neurotoxicity. Moreover, BDNF promotes and controls growth of neo-neurons from neural stem cells (neurogenesis).
Reelin is a large secreted extracellular matrix glycoprotein that helps regulate the process of neuronal migration and positioning in the developing brain by controlling cell-cell interactions. Besides this important role in early development, reelin continues to work in the adult brain. Thus, during embryonic development and adulthood, reelin exerts several critical functions in the brain such as, the regulation of neuronal migration, dendritic growth and branching, dendritic spine formation, as well as synaptogenesis and synaptic plasticity.
Because BDNF and reelin act in concert to regulate many processes during neural development and maintenance, including cell migration and neural plasticity, the question that needs to be addressed is, whether the abnormalities of brain anatomy that had been established might result from neurotropic abnormalities – i.e., abnormalities in those neurochemicals that are significantly involved in constructing and positioning nerve cells in the developing embryo and fetus, and thereafter renewing and maintaining them.
As mentioned earlier, serotonin, which is actively involved in brain-building pre- and postnatally, is also known to express in abnormal levels or quantities in the autistic brain; and GABA, which is a neurotrophins as well as major neuromodulator, has been shown to be depleted or expressed at reduced levels in the brains of individuals with autism. Unlike serotonin and GABA, investigation of the expression levels of BDNF and reelin has not generated consistent findings. As a result, uncertainty remains, regarding the potential role of both BDNF and reelin in the genetic and neurobiological bases of autism.
(xiii) Norepinephrine (aka noradrenalin): Norepinephrine appears to have little or any explanatory value for ASD when considered alone (Figure 7).
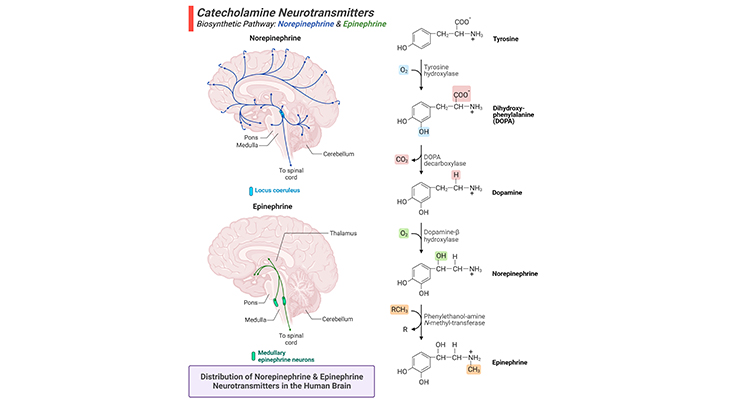
Figure 7. Biosynthetic Pathway and Distribution of Norepinephrine and Epinephrine Neurotransmitters in the Human Brain.
(a) The amino acid tyrosine is the precursor for all three catecholamines, viz., dopamine, norepinephrine, and epinephrine. Dopamine is derived from the amino acid tyrosine. Norepinephrine is produced from dopamine and is a precursor molecule for the hormone epinephrine (right panel). (b) Major projection pathways of the catecholamine neurotransmitter systems in the human brain. Shown are the projections of the norepinephrine and epinephrine systems using the neurotransmitters (left panel). The views are midsagittal sections through the human brain, depicting the medial surface of the right hemisphere; the front pole is at left. In each image, the primary source of the biogenic amine is bold type and colored accordingly.
Nonetheless, the adrenal glands form part of the hypothalamic-pituitary-adrenal axis (HPA). HPA is a major component of neuroendocrine system that governs reactions pertaining to stress, thus regulates many body processes, as next below, cortisol.
(ix) Cortisol: cortisol is a steroid hormone that is secreted by the adrenal glands (which sit on top of each kidney) in response to stress. Variations in cortisol levels are associated with the ‘sleep-wake cycle.’ Observations of vulnerability to anxiety disorders, as well sleep disturbances in children with ASD, have led to some earlier studies of cortisol level. Since then, studies have continued somewhat intermittently, and with mixed findings.
(x) Testosterone: The higher incidence of autism in male individuals might provide valuable clues to the etiology of the condition, which has been described as an ‘extreme of the male brain’ [11]. According to the ‘extreme male brain (EMB)’ theory that postulates exposure to abnormally high levels of testosterone in utero could result in excessively masculinized brain development in individuals, who subsequently diagnosed with ASD. This theory needs further validation from other independent research groups to strengthen the case as hypothesized. However, an ‘extreme male brain’ might throw light on something to sex-specific genetic effects,
- possibly resulting from evolutionary pressures, or
- to the influence of sex hormones on brain development postnatally rather than prenatally, and/or
- a masculinized brain might be attributable to postnatal environmental factors.
In fact, there is some evidence indicating that brain development in females diagnosed with ASD, as well as in males with ASD, resembles brain development in typically developing (TD) males, rather than TD females.
Table 1. Partial list of neurotransmitters organized by chemical properties.
| Neurotransmitters Organized by Chemical Properties | ||
| Monoamines & Acetylcholine | L-Amino Acids | Peptides |
| Dopamine | Glutamate | Enkephalins (endogenous opioid peptides) |
| Norepinephrine | GABA | β-Endorphin (endogenous opioid peptide) |
| Epinephrine | Aspartate | Dynorphins (endogenous opioid peptide) |
| Serotonin | Glycine | Substance P |
| Histamine | Neuropeptide Y (NPY) | |
| Acetylcholine | Peptide YY (PYY) | |
| Orexin (hypocretin) | ||
| Purines | D-Amino Acid | Vasopressin |
| Adenosine | D-Serine | Oxytocin |
| Adenosine triphosphate (ATP) | Corticotrophin releasing hormone (CRH) | |
| Somatostatin | ||
| Gases | Lipids | Neurotensin |
| Vasoactive intestinal polypeptide (VIP) | ||
| Nitric oxide (NO) | Anadamide (endocannabinoid) | Bombesin |
| Carbon monoxide (CO) | 2-Arachidonoylglycerol (endocannabinoid) | Gelanin |
| Vasoactive intestinal polypeptide (VIP) | ||
| Bradykinin | ||
| [Adapted and modified from Hyman SE, Curr Biol. 2005 Mar 8;15(5):R154-8.] | ||
II. Methods of Assessing Brain Chemistry in Subjects with ASD
Earlier research pertaining to neurochemistry in ASD was, however, restricted by the methods available and that consisted of the following:
(1) Post-Mortem or Autopsies Studies: examination of post-mortem brain tissue can reveal whether, for example, the specialized nerve pathways or tracts and receptor cells involved in any one of those neurochemical systems are intact or not. However, the disadvantages are that small numbers of cases/specimens are available, inclined to be older subjects or subjects with multiple disorders that may have affected the brain.
(2) Spinal Tap or Lumbar Puncture (LP): may be employed to draw off a sample of cerebrospinal fluid (CSF) for neurochemical analysis. However, this is an invasive procedure and its use for non-clinical purposes on human subjects is controversial.
(3) Blood and/or Urine Samples: can be instructive about the by-products of brain metabolism.
(4) Efficacy Studies: studies of the effects of medications that are known to have their effects on particular neurotransmitters or neuromodulators provide an indirect method of assessing brain neurochemistry in subjects with ASD.
(5) Animal Models: the efficacy of medications and their potential usefulness to treat undesired behavioral symptoms associated with ASD are generally first tested on animals, usually rodents such as mice or rats.
(6) Insights from Syndromic Forms of ASD: can reveal abnormalities of likely relevance to ASD, for example, phenylketonuria is associated with abnormalities within the serotoninergic systems. (PKU, a rare inherited metabolic disorder that causes an amino acid called phenylalanine to build up in the body)
(7) Magnetic Resonance Spectroscopy Imaging (MRSI/MRIs): similar to structural MRI (sMRI), this noninvasive neuroimaging procedure provides information about the nature of chemical constituents of specific brain regions.
(8) Positron Emission Tomography (PET Scan): utilizes a ‘radioactive tracer’ injected into the blood stream, and the progress of which can be monitored by the scanner. Similar to fMRI, this measures changes in blood flow to a particular region of the brain, indicating the levels of activity in specific neurochemical systems. It is routinely used clinically, though is undesirable for research purposes because of radiation risks.
Summary and Conclusions
In this blog we have provided an overview of the neurochemistry of the autistic brain and described how the abnormalities in the levels of some commonly expressed neurotransmitters can eventually lead to structural and functional brain changes in individual with autism.
First, it is evident that certain neurochemicals are critically involved in the neurobiological basis of ASD. In particular, it is likely that abnormalities affecting the neurotrophic activities of serotonin and GABA, both pre- and postnatally (before- and after-birth) contribute significantly to the atypicalities of brain growth and structure as described in our previous blog (cf. ‘The Neurotypical Brain versus the Autistic Brain: Brain Structure in ASD’).
Secondly, in their neurotransmitter and neuromodulator roles, abnormalities in the production and/or transportation and/or reception of both serotonin and GABA in all probability exert further lifelong effects on brain function and consequently on behavior in individuals with ASD.
Thirdly, the dopaminergic system is unequivocally affected in subjects with ASD. Moreover, reduced expression of GABA would allow the excitatory activities of glutamate to turn unusually dominant in the brain of people with ASD.
Next, the roles that the other neurochemical identified above may play in the causal chain leading to autism-related behavior are less obvious.
Finally, since a secure understanding of the neurochemistry of autism or of subsets within the spectrum would provide a sound basis for the development and testing of drug and dietary treatments for autism, in this context, further research in this emerging field of neurochemistry of autism is of potential practical importance and would warrant further investigation.
For information on autism monitoring, screening and testing please read our blog.
References
- Hyman SE. Neurotransmitters. Curr Biol. 2005 Mar 8;15(5):R154-8. doi: 10.1016/j.cub.2005.02.037. PMID: 15753022.
https://pubmed.ncbi.nlm.nih.gov/15753022/ - Chugani DC. Neurotransmitters. Autism Spectrum Disorders. David Amaral (ed.) et al. Oxford University Press. 2011 May 568-575.
https://doi.org/10.1093/med/9780195371826.003.0037
- Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016 May 3;321:24-41. doi: 10.1016/j.neuroscience.2015.11.010. Epub 2015 Nov 11. PMID: 26577932; PMCID: PMC4824539.
https://pubmed.ncbi.nlm.nih.gov/26577932/ - Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014 Feb 1;86:28-34. doi: 10.1016/j.neuroimage.2013.01.045. Epub 2013 Jan 28. PMID: 23370056; PMCID: PMC3773530.
https://pubmed.ncbi.nlm.nih.gov/23370056/ - Kriete T, Noelle DC. Dopamine and the development of executive dysfunction in autism spectrum disorders. PLoS One. 2015 Mar 26;10(3):e0121605. doi: 10.1371/journal.pone.0121605. PMID: 25811610; PMCID: PMC4374973.
https://pubmed.ncbi.nlm.nih.gov/25811610/ - Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001 Jul;158(7):1058-66. doi: 10.1176/appi.ajp.158.7.1058. PMID: 11431227.
https://pubmed.ncbi.nlm.nih.gov/11431227/
- Alvares GA, Quintana DS, Whitehouse AJ. Beyond the hype and hope: Critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 2017 Jan;10(1):25-41. doi: 10.1002/aur.1692. Epub 2016 Sep 21. PMID: 27651096.
https://pubmed.ncbi.nlm.nih.gov/27651096/ - Carter CS. The Oxytocin-Vasopressin Pathway in the Context of Love and Fear. Front Endocrinol (Lausanne). 2017 Dec 22;8:356. doi: 10.3389/fendo.2017.00356. PMID: 29312146; PMCID: PMC5743651.
https://pubmed.ncbi.nlm.nih.gov/29312146/ - Kasarpalkar NJ, Kothari ST, Dave UP. Brain-Derived Neurotrophic Factor in children with Autism Spectrum Disorder. Ann Neurosci. 2014 Oct;21(4):129-33. doi: 10.5214/ans.0972.7531.210403. PMID: 25452672; PMCID: PMC4248479.
https://pubmed.ncbi.nlm.nih.gov/25452672/ - Hernández-García I, Chamorro AJ, Ternavasio-de la Vega HG, Carbonell C, Marcos M, Mirón-Canelo JA. Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies. Int J Environ Res Public Health. 2020 Oct 30;17(21):8010. doi: 10.3390/ijerph17218010. PMID: 33143244; PMCID: PMC7663127
https://pubmed.ncbi.nlm.nih.gov/33143244/ - Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002 Jun 1;6(6):248-254. doi: 10.1016/s1364-6613(02)01904-6. PMID: 12039606.
https://pubmed.ncbi.nlm.nih.gov/12039606/