Figure 1. Cracking the Folate Code: How Enzymatic Polymorphisms Shape Health and Neurodevelopment. Folate metabolism is a cornerstone of human development, and its genetic underpinnings are increasingly recognized as key contributors to both rare and common disorders—including emerging links to neurodevelopmental conditions such as autism spectrum disorder (ASD).
Introduction
Folate, in its biologically active form tetrahydrofolate (THF), has long been recognized as essential to human health. Its role in preventing megaloblastic anemia was established as early as the 1930s, and by the 1960s, folate supplementation was proposed as a preventive measure against neural tube defects (NTDs). While severe inborn errors of folate metabolism—such as those causing homocystinuria—are rare and typically result from deletions or deleterious mutations that abolish enzyme function, their dramatic clinical consequences have underscored the critical importance of this metabolic pathway [1-3].
Folate metabolism encompasses a network of enzymes responsible for folate absorption, retention, and interconversion of one-carbon (1C) substituted folates. These reactions are central to nucleotide and amino acid biosynthesis, as well as to the methylation cycle. Of particular significance is the remethylation of homocysteine (Hcy) to methionine, which not only detoxifies Hcy but also generates S-adenosylmethionine (SAM)—the universal methyl donor required for DNA methylation, neurotransmitter synthesis, membrane phospholipid remodeling, and creatine production. Disruption of this cycle can have far-reaching effects on cellular function, gene expression, and neurodevelopment.
Over the past two decades, attention has shifted from rare monogenic disorders to more subtle but widespread genetic polymorphisms in folate-related enzymes. These variants, defined by allele frequencies exceeding 1% in the population, may not cause overt disease but can modulate enzyme activity, folate status, and susceptibility to complex conditions. The most extensively studied of these is the 677C→T polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene, first identified in 1995 as a risk factor for vascular disease and later linked to NTDs. Since then, MTHFR and other folate-related gene variants have been implicated in a wide array of multifactorial disorders, including cardiovascular disease, cancer, psychiatric illness, and reproductive complications (see Figure 1).
Notably, emerging evidence suggests a potential link between folate pathway polymorphisms and autism spectrum disorder (ASD). Meta-analyses and clinical studies have associated the MTHFR 677TT genotype with increased ASD risk, possibly through mechanisms involving impaired methylation, elevated homocysteine, and altered neurodevelopmental signaling. Additionally, variants in folate transport genes such as SLC19A1 and the presence of folate receptor alpha autoantibodies have been associated with cerebral folate deficiency in children with ASD, further supporting a role for disrupted folate metabolism in neurodevelopmental vulnerability.
In this article, we provide a comprehensive overview of the most commonly investigated polymorphisms in folate metabolism. We examine their biochemical and metabolic consequences, population prevalence, and associations with human health outcomes—including their emerging relevance to neurodevelopmental disorders. By synthesizing current evidence, we aim to clarify the clinical significance of these variants and highlight opportunities for personalized nutritional and therapeutic strategies.
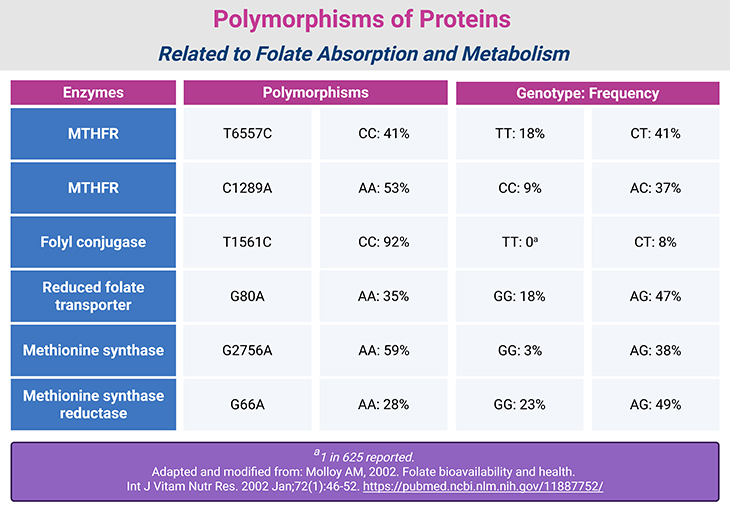
Table 1. Polymorphisms of Proteins Related to Folate Absorption and Metabolism.
Genetic Variation in Folate Metabolism: Definitions and Distinctions
At the heart of genetic diversity lies mutation—the original source of all DNA variation. Yet the terminology used to describe these differences varies across disciplines. In population genetics, a polymorphism refers to a DNA sequence variation where two or more alleles exist at a frequency of at least 1%. Medical geneticists, however, often reserve the term mutation for changes that cause disease, contrasting it with polymorphisms that appear functionally neutral.
Some disease-associated mutations, such as the cystic fibrosis allele in Europeans or β-globin variants in African populations, occur at frequencies above 1%, technically qualifying as polymorphisms. Variants occurring below this threshold are sometimes called variants, though this term is also used broadly to describe any DNA difference, regardless of frequency or effect. Increasingly, the term single nucleotide variant (SNV) is favored over single nucleotide polymorphism (SNP) to avoid ambiguity.
For clarity, this discussion adopts the following definitions:
- Polymorphism (including SNP): ≥1% allele frequency
- Variant: For any less frequent difference between genome copies
- Mutation: A newly arisen (de novo) change
Enzymatic Polymorphisms in Folate Pathways
Genetic variants have been identified in nearly all major enzymes involved in folate metabolism. These polymorphisms influence folate status, homocysteine levels, and disease susceptibility (see Table 1) [4-6].
- Folyl Conjugase: A T→C substitution at base pair 1561 affects folate retention. Individuals with the T allele tend to have higher circulating folate than those with the C allele.
- Reduced Folate Carrier (RFC): A G→A substitution at base pair 80 is associated with elevated homocysteine and reduced erythrocyte folate in individuals homozygous for the G allele.
- Methylenetetrahydrofolate Reductase (MTHFR): Three polymorphisms have been identified:
- C677T: {Some 89% of Americans have the C allele. Some 20% of Mexican Americans, 12% of non-Hispanic whites, but only 1% of non-Hispanic blacks are homozygous for the T variant (TT)}.
This variant significantly reduces enzyme activity—by approximately 70% in TT homozygotes. In addition, TT variant exhibits lower affinity for the flavin cofactor, and lower thermal stability than the C/C form of the enzyme. It is most prevalent in Mexican Americans (20%) and least common in non-Hispanic Blacks (1%) [4-5].
TT individuals exhibit:
- Lower plasma folate
- Elevated plasma 5,10-methylene-THF
- Mild hyperhomocysteinemia
- Reduced global DNA methylation. These effects are exacerbated by low folate intake.
TT genotype is associated with increased risk of:
- Colorectal and lung cancers
- Unipolar depression
- Adult acute lymphoblastic leukemia
- Ischemic stroke
- Cleft lip/palate
- Down syndrome (especially when combined with methionine synthase reductase mutations)
Interestingly, the 677T allele may confer reduced susceptibility to hepatitis B virus (HBV) infection in endemic regions. Mothers and fetuses with the heterozygous CT genotype appear to have higher chances of viable pregnancies and live births. TT individuals also show stronger homocysteine-lowering responses to folate supplementation (see Figure 2; Table 2).
- A1298C: Over 90% of Americans carry the A allele. The C allele alone has minimal physiological impact, but when combined with C677T, it reduces MTHFR activity and folate levels, raising homocysteine. Women with the CC genotype have lower odds of producing healthy infants compared to those with AA.
- G1793A: This polymorphism is rare (~4% prevalence in American women with GG genotype). Its functional significance remains unclear.
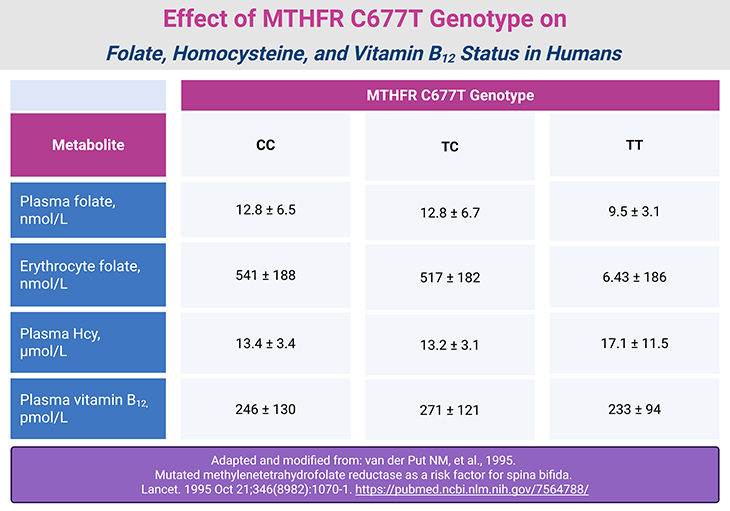
Table 2. Effect of MTHFR C677T Genotype on Folate, Homocysteine, and Vitamin B12 Status in Humans.
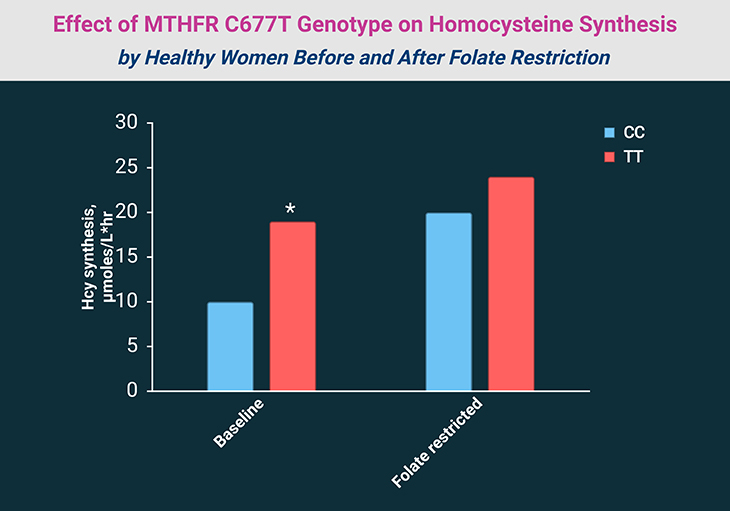
Figure 2. Effect of MTHFR C677T Genotype on Homocysteine Synthesis by Healthy Women Before and After Folate Restriction (115 μg/d for 7 weeks). *P < 0.05. [Adapted and modified from: Quinlivan EP, et al., 2005. Methylenetetrahydrofolate reductase 677C->T polymorphism and folate status affect one-carbon incorporation into human DNA deoxynucleosides. J Nutr. 2005 Mar;135(3):389-96. https://pubmed.ncbi.nlm.nih.gov/15735068/]
- C677T: {Some 89% of Americans have the C allele. Some 20% of Mexican Americans, 12% of non-Hispanic whites, but only 1% of non-Hispanic blacks are homozygous for the T variant (TT)}.
- 10-Formyl-THF Dehydrogenase: Two intronic SNVs have been identified. One is linked to increased breast cancer risk in postmenopausal women. The enzyme is known to be epigenetically silenced in various cancers.
- Dihydrofolate Reductase (DHFR): Three variants affect folate dynamics:
- 19 bp Intron Deletion: Homozygotes show disrupted tissue folate stores and a twofold increase in risk of spina bifida in offspring.
- 5′ Upstream 9 bp Repeat: Associated with low plasma homocysteine but normal circulating folate levels.
- 3′ Untranslated Region Polymorphism: Also linked to low homocysteine with normal folate status.
- Methionine Synthase: An A→G substitution at base pair 2756 alters the B12 methylation domain. The GG genotype lowers homocysteine but raises risk for:
- Systemic lupus erythematosus
- Bipolar disorder
- Schizophrenia
- Congenital facial and spinal malformations
- Down syndrome
- Methionine Synthase Reductase: An A→G substitution at base pair 66 affects ~30% of individuals. Alone, it has little impact, but when paired with MTHFR 677TT, it reduces homocysteine levels by ~26%.
Take-Home Messages
- Folate metabolism is foundational to DNA synthesis, methylation, and neurodevelopment. Disruptions can affect everything from cellular repair to neurotransmitter balance.
- Common genetic polymorphisms—especially in MTHFR, DHFR, and methionine synthase—modulate enzyme activity and folate status. Their subtle effects may influence risk for birth defects, cardiovascular disease, cancer, and neurodevelopmental conditions.
- Emerging evidence links folate pathway variants and folate receptor autoantibodies to autism spectrum disorder (ASD). These may impair methylation and cerebral folate transport, with therapeutic implications for folinic acid supplementation.
- Gene-nutrient interactions matter. The impact of folate-related variants may depend on levels of riboflavin, choline, betaine, and vitamin B12—especially during periods of physiological stress.
- Population-wide screening and personalized nutrition strategies could transform outcomes. Understanding folate genetics empowers targeted interventions for vulnerable groups, including expectant mothers and neurodiverse children.
- As genome-wide technologies expand, so will our ability to decode folate-related risk. Integrating biochemical, genetic, and environmental data is key to unlocking the full potential of precision health.
(Cf. previous blogs entitled as: “Folate in Health and Disease: Genetic Defects in Folate Pathway Influencing the Central Nervous System.”; “Folate in Health and Disease: Folate Concentrations in Serum, Whole Blood, and Cerebrospinal Fluid.”)
Summary and Conclusions
Genetic variation in folate metabolism has emerged as a critical area of investigation, not only for its role in rare hereditary disorders but for its far-reaching implications in common, multifactorial conditions such as cardiovascular disease, birth defects, cancer, and neurodevelopmental disorders. The identification of polymorphisms in folate-related enzymes—particularly MTHFR, DHFR, and methionine synthase—has prompted hundreds of studies annually, yet the clinical significance of these variants remains challenging to confirm due to confounding variables, small cohort sizes, and population heterogeneity.
To advance our understanding, it is essential that future research integrates biochemical characterization of each variant’s impact on enzyme function with in vivo modeling of physiological outcomes. The advent of genome-wide association technologies has accelerated the discovery of novel polymorphisms, but their interpretation demands a careful accounting of both genetic and nongenetic factors—including nutrient status. Folate-related variants do not operate in isolation; their effects may be modulated by levels of riboflavin, choline, betaine, and vitamin B12, as well as by gene-gene interactions. Moreover, the phenotypic consequences of these variants may only emerge under conditions of physiological stress, such as rapid growth, inflammation, or pharmacologic challenge.
Despite these complexities, it is increasingly clear that certain folate pathway variants exert measurable effects on human health. The persistence of deleterious alleles at relatively high frequencies in the population raises intriguing evolutionary questions. It is possible that under specific environmental or nutritional conditions, these variants may confer subtle adaptive advantages—an idea that warrants further exploration through longitudinal and mechanistic studies.
Importantly, the relevance of folate metabolism to the autism spectrum disorder (ASD) community is gaining recognition. The MTHFR 677TT genotype has been associated with increased ASD risk, potentially through impaired methylation capacity, elevated homocysteine, and altered neurodevelopmental signaling. Additionally, folate receptor alpha autoantibodies—detected via FRAT testing—have been linked to cerebral folate deficiency in children with ASD, with promising therapeutic responses to folinic acid supplementation. These findings underscore the need for personalized approaches to folate support in neurodiverse populations and highlight the potential of genetic screening to inform early intervention strategies.
In sum, the study of folate-related polymorphisms offers a powerful lens through which to understand the interplay between genetics, nutrition, and health. As research evolves, it holds promise not only for reducing the burden of common diseases but also for illuminating the biological underpinnings of neurodevelopmental diversity—empowering families, clinicians, and researchers alike.
Did You Know? Folate Receptor Autoantibodies (FRAAs) may impede proper folate transport.
Folate (vitamin B9) is very important for your child’s brain development!
During pregnancy, it helps prevent neural tube defects and plays a big role in forming a normal and healthy baby’s brain and spinal cord. Folate also helps cells divide and assists in both DNA and RNA synthesis.
Emerging research suggests that the presence of FRAAs negatively impacts folate transport into the brain.
- Recent studies reveal that a large subgroup of children with autism spectrum disorder (ASD) have FRAAs.
- This suggests that a possible disruption in folate transport across the blood-cerebrospinal fluid (CSF) barrier may potentially influence ASD-linked brain development.
- Screening for the FRAAs in your child should be part of your early intervention strategies.
Is there a test for identifying Folate Receptor Autoantibodies (FRAAs)?
Yes, there is a test – The Folate Receptor Antibody Test (FRAT®) has emerged as a diagnostic tool for detecting the presence of FRAAs.
It is important to screen at an early age or as soon as possible as there may be corrective measures available. Please consult your physician for further information.
To request a test kit, click on the button below.

For information on autism monitoring, screening and testing please read our blog.
References
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011 Mar;3(3):370-84. doi: 10.3390/nu3030370. Epub 2011 Mar 15. PMID: 22254102; PMCID: PMC3257747. https://pubmed.ncbi.nlm.nih.gov/22254102/
- Li J, Duan H, Ramaswamy H, Wang C. A Comprehensive Review of Fortification, Bioavailability, and Health Benefits of Folate. Int J Mol Sci. 2025 Aug 9;26(16):7703. doi: 10.3390/ijms26167703. PMID: 40869024; PMCID: PMC12386915. https://pubmed.ncbi.nlm.nih.gov/40869024/
- Ramaekers VT, Quadros EV. Cerebral Folate Deficiency Syndrome: Early Diagnosis, Intervention and Treatment Strategies. Nutrients. 2022 Jul 28;14(15):3096. doi: 10.3390/nu14153096. PMID: 35956272; PMCID: PMC9370123. https://pubmed.ncbi.nlm.nih.gov/35956272/
- Wang M, Zheng Q, You L, Wang H, Jia P, Liu X, Zeng C, Xu G. Quantification of multi-pathway metabolites related to folate metabolism and application in natural population with MTHFR C677T polymorphism. Anal Bioanal Chem. 2025 May;417(13):2807-2821. doi: 10.1007/s00216-024-05688-w. Epub 2024 Dec 18. PMID: 39690314.
https://pubmed.ncbi.nlm.nih.gov/39690314/ - Mansoor MA, Stea TH, Slettan A, Perera E, Maddumage R, Kottahachchi D, Ali DS, Cabo R, Blomhoff R. Impact of single nucleotide polymorphisms (SNPs) in antioxidant-enzyme genes on the concentrations of folate, homocysteine and glutathione in plasma from healthy subjects after folic acid supplementation – a randomized controlled crossover trial. Genes Nutr. 2025 Jan 21;20(1):1. doi: 10.1186/s12263-024-00761-6. PMID: 39838297; PMCID: PMC11752798.
https://pubmed.ncbi.nlm.nih.gov/39838297/ - James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006 Dec 5;141B(8):947-56. doi: 10.1002/ajmg.b.30366. PMID: 16917939; PMCID: PMC2610366.
https://pubmed.ncbi.nlm.nih.gov/16917939/