Table of Contents
- Introduction
- MTHFR Polymorphisms at the Nexus of Metabolism, Development, and Disease
- MTHFR 677C→T: A Thermolabile Variant at the Heart of One-Carbon Metabolism
- Clinical Implications of MTHFR 677C→T Variant
- MTHFR 1298A→C: A Subtler Genetic Player
- Clinical Relevance of MTHFR 1298A→C Variant
- Final Reflection
- Take-Home Messages
- Summary and Conclusions
- Did You Know About Folate Receptor Autoantibodies (FRAAs) and Brain Development?
- References
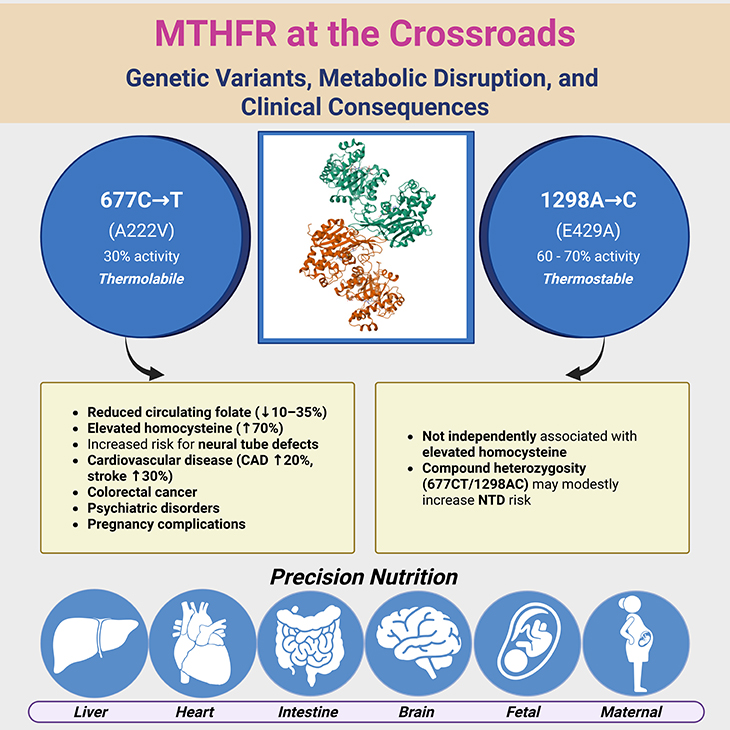
Figure 1. MTHFR at the Crossroads: Genetic Variants, Metabolic Disruption, and Clinical Consequences. This infographic illustrates the central role of MTHFR in folate metabolism and the clinical impact of its two common polymorphisms: 677C→T (A222V) and 1298A→C (E429A). The top center panel depicts the three-dimensional (3D) structure of human 5,10-methylenetetrahydrofolate reductase (MTHFR; PDB ID: 6FCX) and its role in folate metabolism; that is irreversible conversion of 5,10-methylene-THF to 5-methyl-THF, catalyzed by MTHFR with cofactors FAD and NADPH—a critical step that commits one-carbon units (1C) to the methylation cycle, influencing homocysteine remethylation, DNA synthesis, and epigenetic regulation. The top left panel highlights the 677C→T variant, which produces a thermolabile enzyme with only ~30% residual activity in TT homozygotes. This leads to elevated homocysteine (↑70%), reduced circulating folate (↓10–35%), and increased risk for neural tube defects, cardiovascular disease (CAD ↑20%, stroke ↑30%), colorectal cancer, psychiatric disorders, and pregnancy complications—especially under conditions of low folate or riboflavin. The top right panel summarizes the 1298A→C variant, which modestly reduces enzyme activity (60–70% of wild-type) without thermolability. It is not independently associated with elevated homocysteine or major disease risk, though compound heterozygosity (677CT/1298AC) may modestly increase NTD risk in select populations. System icons below represent the variant-specific ripple effects across vascular, neurological, reproductive, oncologic, and methylation-dependent pathways, reinforcing the clinical relevance of MTHFR polymorphisms in precision medicine. [Adapted and modified from: https://www.rcsb.org/structure/6FCX].
Introduction
MTHFR Polymorphisms at the Nexus of Metabolism, Development, and Disease
In the intricate choreography of human metabolism, few enzymes command as central a role as methylenetetrahydrofolate reductase (MTHFR). By catalyzing the irreversible reduction of 5,10-methylene-THF to 5-methyl-THF, MTHFR commits one-carbon (1C) units to the methylation cycle—an essential pathway for DNA synthesis, epigenetic regulation, and homocysteine remethylation. This reaction, dependent on flavin adenine dinucleotide (FAD) and nicotinamide-adenine dinucleotide phosphate, reduced form (NADPH), is not merely biochemical—it is biologically decisive.
The discovery of the MTHFR 677C→T polymorphism, a thermolabile variant associated with elevated plasma homocysteine (Hcy), marked a turning point in our understanding of folate metabolism and its clinical implications. Since its initial identification in the context of homocystinuria, this variant has become the most extensively studied genetic alteration in the folate pathway. Its impact reverberates across disciplines—from cardiovascular medicine and oncology to neurodevelopment, reproductive health, and pharmacogenetics (see Figure 1).
Alongside 677C→T, the MTHFR 1298A→C variant—though biochemically subtler—adds complexity to the genotype–phenotype landscape. Together, these polymorphisms shape the distribution of folate derivatives, influence methylation capacity, and modulate disease risk in ways that are ethnically variable, nutrient-sensitive, and clinically nuanced.
This synthesis explores the molecular architecture, biochemical consequences, and health implications of MTHFR 677C→T and 1298A→C, integrating recent findings and highlighting the interplay between genetic variation, nutrient status, and clinical outcomes. In doing so, it offers a comprehensive narrative for clinicians, researchers, and public health professionals seeking to translate genetic insights into actionable strategies [1].
I. MTHFR 677C→T: A Thermolabile Variant at the Heart of One-Carbon Metabolism
- Catalytic Role and Discovery
The enzyme methylenetetrahydrofolate reductase (MTHFR) plays a pivotal role in the irreversible reduction of 5,10-methylene-THF to 5-methyl-THF, a critical step that commits one-carbon (1C) units to the methylation cycle. This reaction depends on the cofactors flavin adenine dinucleotide (FAD) and reduced nicotinamide-adenine dinucleotide phosphate (NADPH) (see Figure 2). Early investigations into rare MTHFR mutations linked to homocystinuria led to the identification of a thermolabile variant, now known as MTHFR 677C→T, which was subsequently associated with elevated plasma homocysteine (Hcy) and proposed as a risk factor for cardiovascular disease. Since its initial characterization, this variant has become the most extensively studied polymorphism in folate metabolism [2-3].
- Genetic Identity and Global Prevalence
The MTHFR 677C→T variant (dbSNP ID: rs1801133) is a nonsynonymous mutation in exon 4, resulting in an alanine-to-valine substitution (A222V) within the enzyme’s catalytic domain. Depending on the reference sequence, this mutation is alternatively numbered as 665C→T (NM_005957.3) or 677C→T (U09806.2).
The 677TT genotype exhibits striking ethnic and geographic variability:
- North American Whites: 8–14%
- Northern Europeans: 6–14%; Southern Europeans: 15–24%
- Mexican and Hispanic populations: 15–35%
- African and African American populations: <2%
- Asian populations: 12–18%
- Biochemical Consequences of the 677C→T Substitution
The 677C→T substitution produces a thermolabile enzyme with markedly reduced activity:
- After heating at 46°C, the variant retains only ~30% of wild-type activity.
- In vivo, lymphocyte extracts from TT individuals show ~30% activity compared to CC homozygotes.
Although the purified A222V protein maintains catalytic function in vitro, it exhibits a greater propensity to lose its FAD cofactor, compromising stability. Supplementation with methyl-THF and/or FAD can mitigate thermolability and protect against FAD dissociation. Interestingly, S-adenosylmethionine (SAM), while not affecting enzyme inhibition, also stabilizes the variant by reducing FAD loss (see Figure 2).
- Impact on Homocysteine and Folate Metabolism
As MTHFR is the sole source of 5-methyl-THF, reduced activity disrupts the methylation of Hcy, leading to elevated plasma homocysteine—up to 70% higher in TT individuals compared to CC homozygotes, especially under low folate conditions. This genotype–folate interaction reflects the folate-dependent stabilization of the variant enzyme.
Additional nutrient interactions exacerbate this effect:
- Low riboflavin (FAD precursor) further increases Hcy in TT individuals.
- Vitamin B12 deficiency, a cofactor for methionine synthase (MTR), amplifies Hcy elevation under low folate.
- Reduced plasma betaine in TT individuals suggests increased reliance on betaine-homocysteine methyltransferase (BHMT) for Hcy remethylation.
- Redistribution of Folate Derivatives and Nucleotide Synthesis
Reduced MTHFR activity alters the intracellular folate landscape:
- Methyl-THF decreases, while methylene-THF and formyl-THF increase.
- These shifts promote dTMP synthesis and de novo purine biosynthesis.
- TT individuals show elevated formyl-THF in red blood cells (RBCs)—a folate species not typically present—alongside reduced methyl-THF, the primary transport form.
Consequently, circulating folate levels decline, with TT individuals exhibiting a 10–35% reduction in plasma/serum folate. Measurement of RBC folate is confounded by altered folate distributions and assay limitations, whereas plasma folate assays remain reliable.
- Methylation Disruption and Epigenetic Implications
Reduced MTHFR activity may impair methylation reactions due to:
- Decreased SAM (methyl donor)
- Increased S-adenosylhomocysteine (SAH), a potent methyltransferase inhibitor
These disruptions have been linked to:
- DNA hypomethylation
- Chromatin remodeling
- Genomic instability, particularly in neoplastic tissues
However, two studies found no significant changes in SAM/SAH ratios or DNA methylation attributable to the 677C→T genotype under variable folate intake. Other methylation-dependent processes—such as choline synthesis and protein methylation—remain underexplored.
- Clinical Implications and Mechanistic Pathways
The MTHFR 677C→T variant may influence disease risk through multiple interrelated mechanisms:
- Elevated homocysteine
- Reduced circulating folate
- Altered folate partitioning
- Abnormal nucleotide synthesis
- Compromised methylation capacity
Together, these biochemical perturbations underscore the variant’s potential role in cardiovascular disease, neurodevelopmental disorders, oncogenesis, and reproductive complications—a topic we will explore further in Part II (continued, see below).
II. Clinical Implications of MTHFR 677C→T Variant
- Birth Defects and Developmental Disorders
The MTHFR 677C→T variant was first linked to neural tube defects (NTDs) in 1995 [4]. Since then, numerous meta-analyses have confirmed that both TT and CT genotypes, whether present in the mother or the child, elevate NTD risk. Reported odds ratios (ORs) range from 1.4 to 10.9 for affected children, and 1.6 to 2.0 for mothers. However, this association is not universally observed, likely due to modifying factors such as:
- Ethnicity
- Specific NTD subtype or anatomical site
- Gene–gene interactions
- Folate status and vitamin supplementation
Importantly, dual TT genotypes in both mother and child may compound risk.
The variant has also been explored in relation to congenital heart defects (CHDs). While findings are mixed, recent meta-analyses suggest weak associations across CHD subtypes. Elevated homocysteine (Hcy) levels may contribute to CHD pathogenesis, and maternal folate intake appears to modulate this risk. The type of CHD and maternal nutritional status are critical variables in interpreting these associations.
Other birth defects—such as cleft lip and palate and Down syndrome—have been investigated with inconclusive results. Again, folate fortification and population-level nutrient status may obscure genotype–phenotype relationships.
- Cardiovascular Disease (CVD)
The 677TT genotype has been extensively studied as a risk factor for coronary artery disease (CAD), stroke, venous thrombosis, and hypertension [5-6]. Meta-analyses indicate:
- A 20% increased risk for CAD, particularly in Asian populations
- A 30% increased risk for stroke
- A 1.2–1.5-fold increased risk for venous thrombosis
These effects are often attenuated in North American populations, likely due to higher folate intake. The variant’s impact is more pronounced in early-onset CAD, consistent with its role as a genetic determinant. Importantly, the absence of other major CAD risk factors may unmask the variant’s contribution.
Population admixture and ethnic mismatches between cases and controls can confound results. Folate status remains a critical modifier, reinforcing the need for nutrient-genotype stratification in risk assessment.
- Colorectal and Other Cancers
Initial reports suggested a protective effect of the TT genotype against colorectal cancer (CRC) [7]. This has been substantiated by meta-analyses showing a ~20% reduction in CRC risk, but only when folate concentrations are high. Under low folate conditions, the TT genotype may lose its protective effect or even become a risk factor.
Mechanistically, this protection may stem from:
- Increased methylene-THF, enhancing dTMP synthesis and reducing uracil misincorporation
- Elevated Hcy or its metabolites, promoting apoptosis in transformed intestinal cells
Modifiers of this effect include:
- Tumor location
- Alcohol intake
- Ethnicity
- Folate status
Interestingly, the variant does not appear to influence colorectal adenoma risk, suggesting a role in preventing adenoma-to-carcinoma progression.
In contrast, the TT genotype may increase gastric cancer risk by 40–50%, with folate status and tumor site again acting as modifiers. Emerging data on breast cancer, leukemia, and other malignancies remain preliminary, and further studies are needed to clarify these associations.
- Neuropsychiatric and Reproductive Health
The TT genotype has been linked to increased risk for:
- Schizophrenia and depression (up to 40% increased risk)
- Pregnancy complications, including recurrent loss, severe hypertension, and preeclampsia
These effects are likely mediated by hyperhomocysteinemia, which disrupts vascular and methylation pathways.
- Pharmacogenetic Implications
The 677C→T variant may influence response to:
- Antifolate chemotherapeutics (e.g., methotrexate, 5-fluorouracil)
- Anticonvulsants
- Other folate-dependent medications
This highlights the variant’s relevance in personalized medicine, particularly in oncology and neurology.
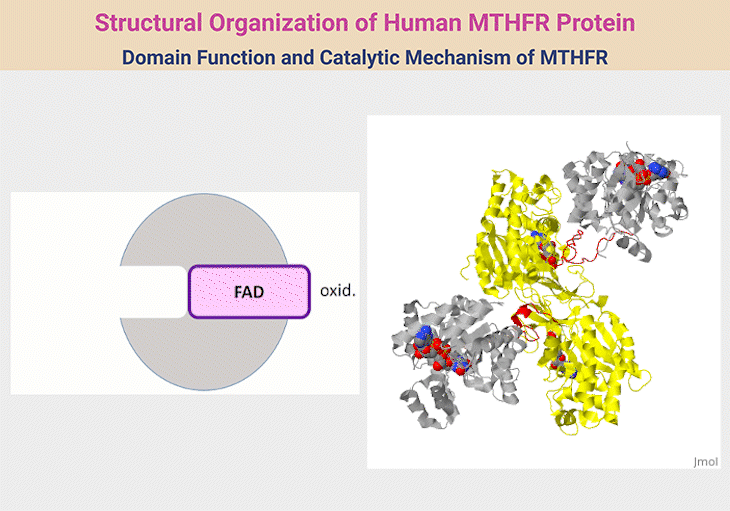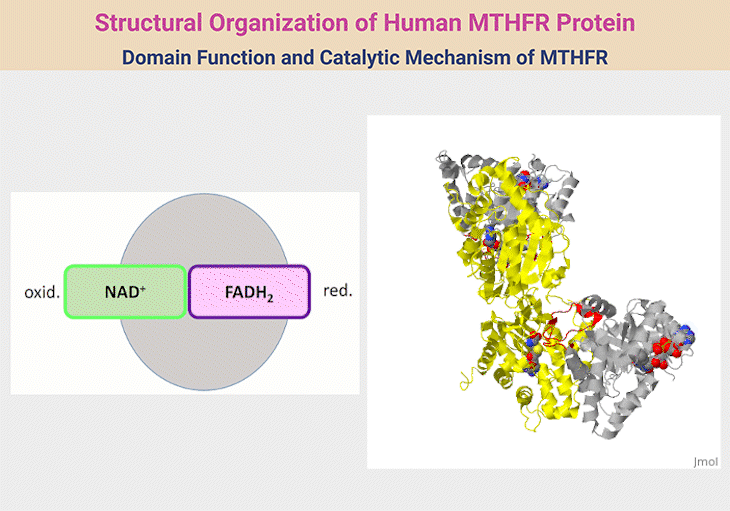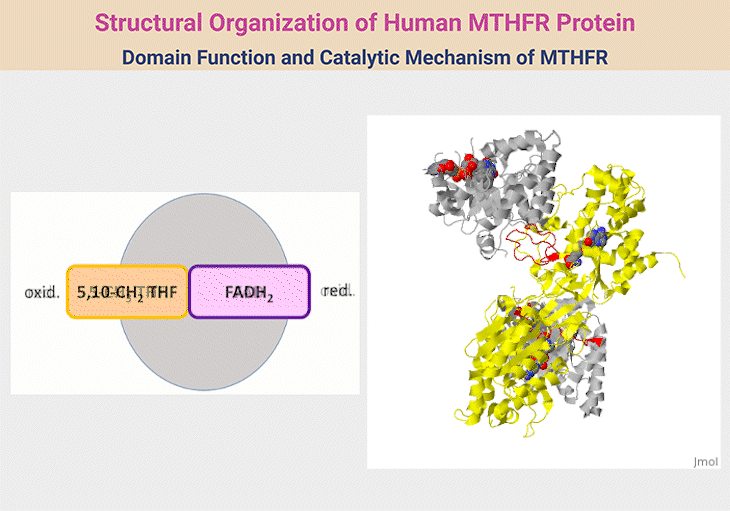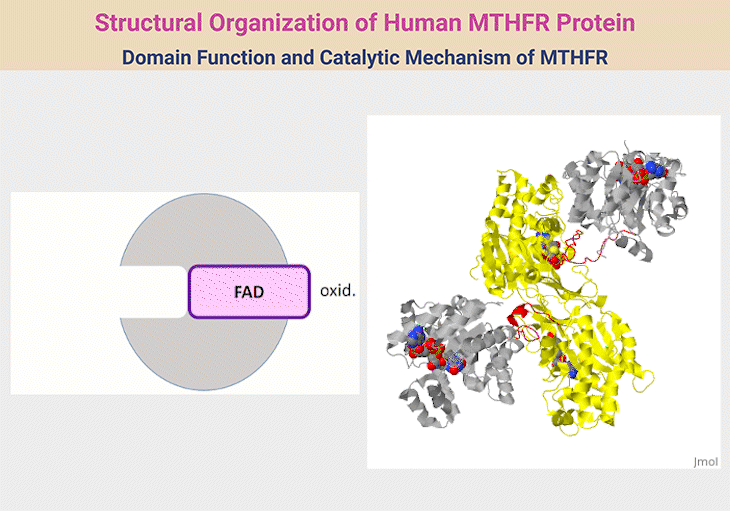
Figure 2. Structural Organization of Human MTHFR Protein. 1. Structural Organization of Human MTHFR. MTHFR functions as a homodimer, with each monomer comprising an N-terminal catalytic domain and a C-terminal regulatory domain, connected by a short linker (red). The catalytic domain (silver) facilitates the conversion of 5,10-methylene-THF to 5-methyl-THF—an essential methyl donor for methionine synthase. This domain houses the cofactor FAD and interacts with NADPH as an electron donor. The regulatory domain (yellow) binds S-adenosylhomocysteine (SAH), enabling the enzyme to sense and respond to cellular methylation demands through activation or inhibition. The reaction proceeds via a ping-pong mechanism, where one substrate binds and forms an intermediate before releasing its product, followed by the sequential binding and release of a second substrate. 2. Domain Function and Catalytic Mechanism of MTHFR. The human MTHFR protein (PDB ID: 6FCX) forms a homodimer, with each monomer comprising an N-terminal catalytic domain (depicted in silver), linked via a short red connector to a C-terminal regulatory domain (shown in yellow). The catalytic region houses the cofactor FAD, essential for enzymatic activity. In this structural model, S-adenosyl homocysteine is bound within the regulatory domain. Both ligands are visualized using space-filling representations in standard CPK coloring. [Adapted and modified from:
https://proteopedia.org/wiki/index.php/Methylenetetrahydrofolate_reductase#:~:text=with%20learning%20disorders.-,Structure,Ligands%20as%20spacefilling]
III. MTHFR 1298A→C: A Subtler Genetic Player
- Genetic Identity and Prevalence
The MTHFR 1298A→C variant (rs1801131) is a nonsynonymous mutation in exon 7, resulting in a glutamate-to-alanine substitution (E429A) within the regulatory domain. Based on reference sequences, it is alternatively numbered as 1286A→C (NM_005957.3).
Prevalence of the 1298CC genotype varies:
- North American and European Whites: 6–11%
- African populations: 2–4%
- Asian populations: 1.4–3.7%
- US Hispanic and Mexican populations: 2–4%
The 677T and 1298C alleles are rarely found in cis configuration, and compound homozygosity is exceedingly rare due to linkage disequilibrium.
- Biochemical Characteristics
Compared to 677C→T, the 1298A→C variant exerts milder biochemical effects:
- 1298CC individuals retain 60–70% of wild-type activity
- The enzyme is not thermolabile
- Catalytic function, SAM inhibition, and FAD release remain unaffected
Most studies report no significant changes in plasma Hcy or folate. However, compound heterozygotes (677CT/1298AC) may exhibit elevated Hcy in some populations, though the 677C→T allele consistently dominates the biochemical phenotype.
IV. Clinical Relevance of MTHFR 1298A→C Variant
The 1298A→C variant is not an independent risk factor for:
- NTDs
- CHDs
- Cleft lip and palate
- Cardiovascular disease
Some studies suggest compound heterozygosity may modestly increase NTD risk, but findings are inconsistent.
Regarding cancer:
- CRC studies show mixed results, with one meta-analysis suggesting a protective effect of the CC genotype
- No significant associations have been found for gastric cancer
Overall, the 1298A→C variant appears to exert subtle effects, often overshadowed by the more impactful 677C→T polymorphism.
Final Reflection
Together, the MTHFR 677C→T and 1298A→C variants illustrate the complex interplay between genetics, nutrition, and disease. While 677C→T is a robust modifier of folate metabolism and clinical risk, 1298A→C serves as a modest contributor, occasionally amplifying effects in compound genotypes. Their influence is shaped by ethnicity, nutrient status, and disease-specific mechanisms, offering a compelling case for precision medicine and nutrigenomic strategies in maternal, cardiovascular, oncologic, and neuropsychiatric health.
Take-Home Messages
- MTHFR is a metabolic gatekeeper, directing one-carbon units toward methylation via the irreversible reduction of 5,10-methylene-THF to 5-methyl-THF. Its function is indispensable for homocysteine remethylation, DNA synthesis, and epigenetic regulation.
- The MTHFR 677C→T polymorphism (A222V) produces a thermolabile enzyme with up to 70% reduced activity in TT homozygotes, leading to hyperhomocysteinemia, altered folate distribution, and impaired methylation capacity, especially under low folate or riboflavin status.
- The 677TT genotype is associated with a 10–35% reduction in circulating folate, a 70% increase in plasma homocysteine, and increased reliance on alternative remethylation pathways (e.g., BHMT), particularly in the context of low folate, B12, or riboflavin.
- Clinical consequences of the 677C→T variant span multiple systems:
- Neural tube defects (NTDs): ORs range from 1.4 to 10.9 in children and 1.6 to 2.0 in mothers, with risk modulated by folate status, ethnicity, and co-inheritance.
- Cardiovascular disease: TT genotype increases risk for CAD (20%), stroke (30%), and venous thrombosis (1.2–1.5×), particularly in low-folate or Asian populations.
- Colorectal cancer: TT genotype may be protective (~20% risk reduction) under high folate, but deleterious under deficiency.
- Gastric cancer: Risk may increase by 40–50%, with folate status and tumor site as modifiers.
- Neuropsychiatric and reproductive disorders: TT genotype is linked to schizophrenia, depression (up to 40% increased risk), and pregnancy complications (e.g., preeclampsia, recurrent loss).
- The MTHFR 1298A→C variant (E429A) exerts modest biochemical effects, with 60–70% residual activity in CC homozygotes. It is not thermolabile, and does not independently affect homocysteine or folate levels in most populations.
- 1298A→C is not a major independent risk factor for NTDs, CHDs, CVD, or most cancers. However, compound heterozygosity (677CT/1298AC) may modestly increase NTD risk in select populations.
- The clinical impact of MTHFR polymorphisms is context-dependent, shaped by ethnicity, nutrient status, co-inherited variants, and environmental exposures. These interactions underscore the importance of precision nutrition and personalized risk assessment.
- Folate sufficiency remains the most effective modifier of MTHFR-related risk. Targeted supplementation, particularly in TT individuals, may mitigate adverse outcomes across developmental, cardiovascular, oncologic, and neuropsychiatric domains.
- As our understanding deepens, MTHFR genotyping offers a window into gene–nutrient–disease interactions, with implications for public health policy, clinical decision-making, and translational research in maternal and child health.
(Cf. previous blogs entitled as: “Cracking the Folate Code: How Enzymatic Polymorphisms Shape Health and Neurodevelopment.”; “Folate in Health and Disease: Genetic Defects in Folate Pathway Influencing the Central Nervous system.”)
Summary and Conclusions
The methylenetetrahydrofolate reductase (MTHFR) enzyme serves as a biochemical fulcrum in one-carbon metabolism, catalyzing the irreversible reduction of 5,10-methylene-THF to 5-methyl-THF—a reaction essential for homocysteine remethylation, DNA synthesis, and methylation-dependent regulation of gene expression. Among the known polymorphisms, MTHFR 677C→T (A222V) and 1298A→C (E429A) have emerged as key genetic variants with distinct biochemical and clinical implications.
The 677C→T variant, characterized by thermolability and reduced enzymatic activity, leads to elevated plasma homocysteine, altered folate distribution, and impaired methylation capacity—particularly under conditions of low folate, riboflavin, or vitamin B12. Its impact spans multiple domains: increased risk for neural tube defects (NTDs), congenital heart defects (CHDs), cardiovascular disease (CAD, stroke, thrombosis), and certain cancers such as colorectal and gastric malignancies. Neuropsychiatric disorders including schizophrenia and depression, as well as pregnancy complications like preeclampsia and recurrent loss, have also been linked to this variant. Importantly, the clinical expression of 677C→T is modulated by ethnicity, nutrient status, and co-inherited genetic variants, underscoring the need for personalized risk assessment.
In contrast, the 1298A→C variant exerts subtler biochemical effects, with no thermolability and modest reductions in MTHFR activity. It is not independently associated with elevated homocysteine or folate deficiency in most populations. While not a major risk factor for NTDs, CHDs, or cardiovascular disease, compound heterozygosity with 677C→T may modestly increase NTD risk. Its role in cancer remains equivocal, with some evidence suggesting a protective effect against colorectal cancer, though findings are inconsistent.
Despite extensive research, several knowledge gaps persist. The precise mechanisms by which MTHFR variants influence epigenetic regulation, chromatin remodeling, and gene–nutrient interactions remain incompletely understood. The impact of these polymorphisms on non-DNA methylation reactions, such as choline synthesis and protein methylation, has yet to be fully explored. Moreover, the influence of compound genotypes, cis/trans configurations, and population admixture on disease risk warrants deeper investigation.
Future research should prioritize:
- Longitudinal cohort studies integrating genotype, nutrient status, and clinical outcomes
- Functional assays to elucidate variant-specific effects on methylation and nucleotide synthesis
- Population-specific risk models that account for dietary patterns, fortification policies, and genetic ancestry
- Pharmacogenomic profiling to optimize antifolate therapies and neuropsychiatric treatments in MTHFR variant carriers
In conclusion, MTHFR polymorphisms—particularly 677C→T—represent a compelling intersection of genetics, nutrition, and disease susceptibility. Their study not only advances our understanding of metabolic regulation but also opens avenues for precision medicine, targeted supplementation, and public health interventions aimed at improving maternal, cardiovascular, oncologic, and neurodevelopmental outcomes across diverse populations.
For information on autism monitoring, screening and testing please read our blog.
References
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011 Mar;3(3):370-84. doi: 10.3390/nu3030370. Epub 2011 Mar 15. PMID: 22254102; PMCID: PMC3257747.
https://pubmed.ncbi.nlm.nih.gov/22254102/
https://www.mdpi.com/2072-6643/3/3/370 - Bailey LB, Gregory JF 3rd. Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: metabolic significance, risks and impact on folate requirement. J Nutr. 1999 May;129(5):919-22. doi: 10.1093/jn/129.5.919. PMID: 10222379.
https://pubmed.ncbi.nlm.nih.gov/10222379/
https://www.sciencedirect.com/science/article/pii/S0022316623020333 - Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015 Jan;58(1):1-10. doi: 10.1016/j.ejmg.2014.10.004. Epub 2014 Nov 4. PMID: 25449138.
https://pubmed.ncbi.nlm.nih.gov/25449138/
https://www.sciencedirect.com/science/article/abs/pii/S1769721214001931?via%3Dihub - Whitehead AS, Gallagher P, Mills JL, Kirke PN, Burke H, Molloy AM, Weir DG, Shields DC, Scott JM. A genetic defect in 5,10 methylenetetrahydrofolate reductase in neural tube defects. QJM. 1995 Nov;88(11):763-6. PMID: 8542260.
https://pubmed.ncbi.nlm.nih.gov/8542260/ - Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 May;10(1):111-3. doi: 10.1038/ng0595-111. PMID: 7647779.
https://pubmed.ncbi.nlm.nih.gov/7647779/ - Moll S, Varga EA. Homocysteine and MTHFR Mutations. Circulation. 2015 Jul 7;132(1):e6-9. doi: 10.1161/CIRCULATIONAHA.114.013311. PMID: 26149435.https://pubmed.ncbi.nlm.nih.gov/26149435/
https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.114.013311 - Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004 Mar 1;159(5):423-43. doi: 10.1093/aje/kwh066. PMID: 14977639.
https://pubmed.ncbi.nlm.nih.gov/14977639/