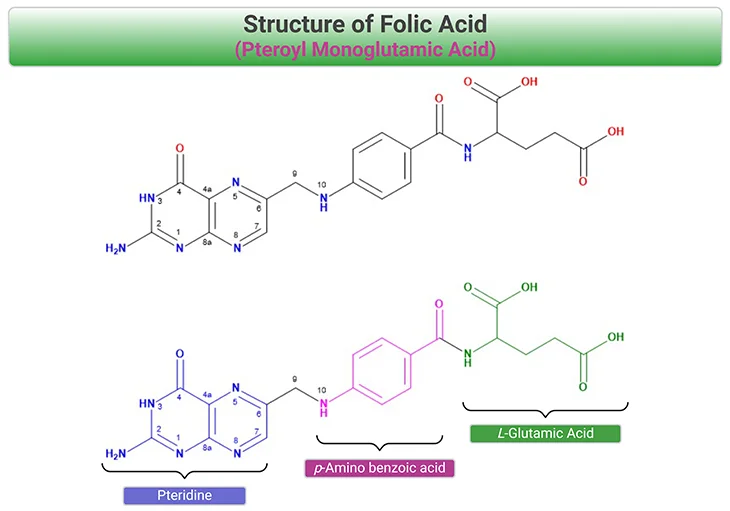
Figure 1. Structure of Folic Acid (FA): Folic acid (pteroylmonoglutamic acid) is the synthetic and the most stable form of the vitamin. FA is fully oxidized and consists of a
2-amino-4-hydroxy-pteridine ring (blue), linked at the C-6 position to a p-aminobenzoic acid (pABA) (fuchsia), and L-glutamic acid (green). FA is the preferred form that is used in dietary supplements and fortified foods, because of its stability and taste and odorless characteristics.
Introduction
Folates represent a large family of water-soluble B vitamins, which were first recognized and reported by Lucy Wills in 1933 as a hematopoietic factor in yeast and liver extracts. In 1940, Snell and colleagues described a factor that is essential for the growth of Lactobacillus casei. Subsequently, this factor was isolated, characterized, and named as ‘folate.’ In 1941, Mitchell coined the term ‘folic acid’ (Latin word ‘folium’ for ‘leaf’), who extracted the substance from 4 tons of spinach leaves [1].
Biochemistry and Function of Folates in Human Cells
By convention, ‘folates’ is a generic term, referring to a large family of compounds consisting of a 2-amino-4-hydroxy-pteridine ring, linked by a methylene (CH2) group to a p-aminobenzoyl moiety, which is in turn linked through amide bond to the α-amino group of a monoglutamate or poly-γ-glutamate. One-carbon (1-C) units can be attached to N-5, N-10, or both. On the other hand, the name ‘folic acid’ is reserved for the synthetic form with the fully oxidized pteridine ring and no 1-C substitution (see Figure 1) [2].
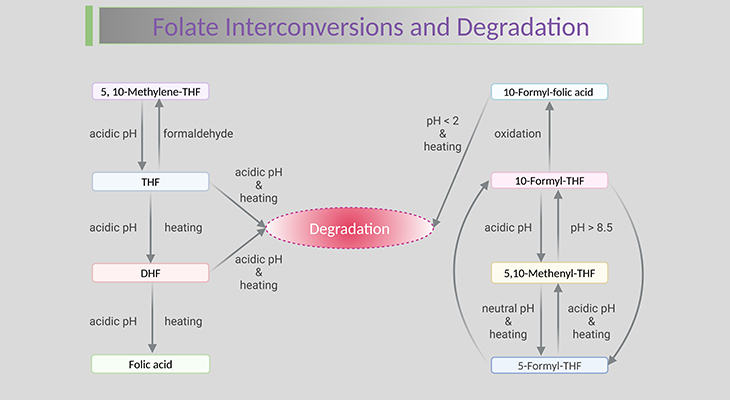
Naturally occurring folate forms consist of derivatives of 5,6,7,8-tetrahydropteroyl-γ-glutamate (THF) that are fully reduced at 5,6,7, and 8 positions of the pyrazine ring. The molecular structure of FA, THF, and the reduced folate forms are shown in Figure 2. Natural folate forms are polyglutamates, with five to eight glutamate residues prevailing in the mammalian cell. Folates exhibit a one-carbon unit at the N-5 and/or N-10 position, which can be transferred by means of certain enzymes.
Reduced folates function as acceptors and donors of ‘one-carbon units’ at three different oxidation levels (see Figure 2) [3], for example:
- methanol (5-methyl-THF),
- formaldehyde (5, 10-methylene-THF), and
- formic acid (5,10-methenyl-THF, 5-formimino-THF, 5-formyl-THF, and 10-formyl-THF).
Over one hundred different vitamers (folate derivatives) has been detected in biological tissues. Vitamers are the members of the same vitamin family; and are chemically related compounds having qualitatively comparable metabolic activities, i.e., similar vitamin activities.
In summary, folate forms differ in three aspects, for instance:
- the hydrogenation of pteridines [either oxidized (FA), di- (7,8-dihydrofolate, DHF), or tetra-hydrated (5,6,7,8-tetrahydrofolate, THF)];
- the degree of substitution of the atoms N-5 and N-10; and
- the number of glutamyl residues that are linked by γ-peptide bonds.
These chemical differences are strongly related to the bioavailability, cellular distribution, and functions of the different folate forms.
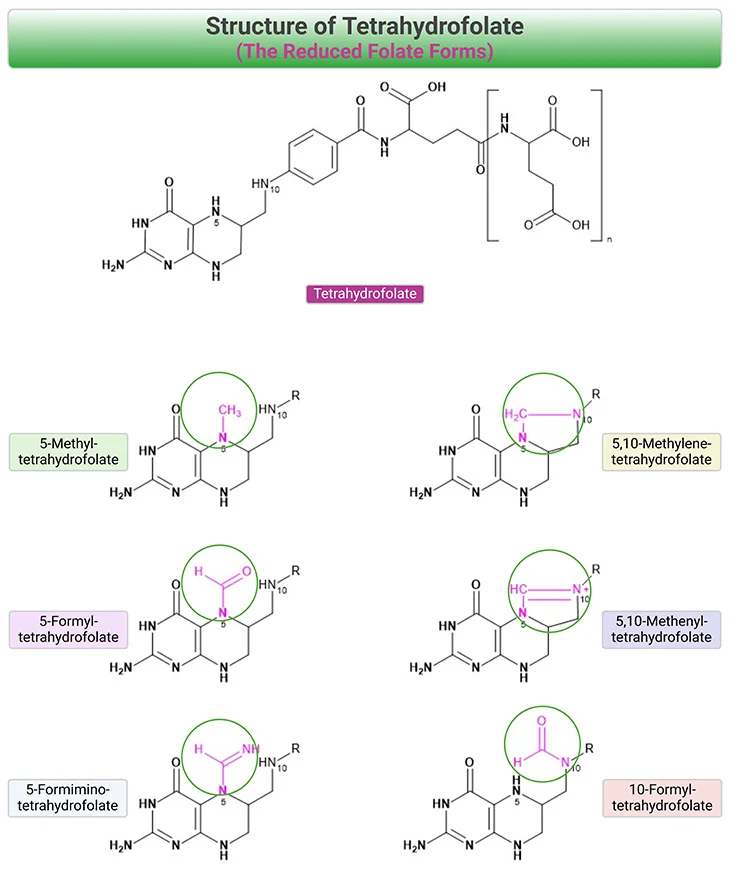
Figure 2. Structure of Tetrahydrofolate (THF) and the Reduced Folate Forms. Bonds at positions, 5,6,7, and 8 can be oxidized to 5,6-dihydrofolate (DHF). One carbon units can be accepted by tetrahydrofolate (THF) at N-5 and/or N-10 positions of the pteridine ring.
Adequate folate intake is crucial for cell division and homeostasis. Folates act as essential co-enzymes in many biological pathways (see Figure 3), such as:
- purine and thymidylate biosynthesis,
- deoxyribonucleic acid (DNA) methylation,
- serine and glycine metabolism, and
- the generation of methionine.
The folate metabolism intersects with the methionine cycle and the choline pathway.
Apart from DNA synthesis, important functions are the methylation of homocysteine (Hcy) and the formation of S-adenosyl methionine (SAM), the most important methyl donor in the cell. Most of the folate coenzymes exist in the liver (hepatic folate levels range from 10 – 35 μmol/L).
The dihydrofolate reductase (DHFR) reduces FA to DHF and DHF to the active form, THF. The cytosolic folate metabolism consists of three interrelated cycles (see Figure 3), viz.,
- One cycle starts from 10-formyl-THF and produces purines, and
- Two cycles use 5, 10-methylene-THF to form deoxythymidine monophosphate (dTMP) and methionine.
5-Methyl-THF is the predominant folate form, comprising 82-93% of total folate in the human serum. After cellular uptake, 5-methyl-THF is converted into THF. This reaction is carried out by the cobalamin (Cbl, vitamin B12)-dependent methionine synthase.
The methionine cycle is an important pathway for the conversion of Hcy (a non-proteinogenic amino acid) to methionine (an essential proteinogenic amino acid) and the formation of SAM. Elevated plasma-concentrations of total Hcy (tHcy) can be caused by deficiencies of B vitamins [such as vitamin B9 (folate), vitamin B12 (Cbl), or vitamin B6 (pyridoxal 5’-phosphate)] or genetic defects. 5, 10-Methylene-THF is converted to
5-methyl-THF by the flavoprotein 5,10-methylene-tetrahydrofolate reductase (MTHFR) in an irreversible and flavin adenine dinucleotide (FADH) and nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reaction.
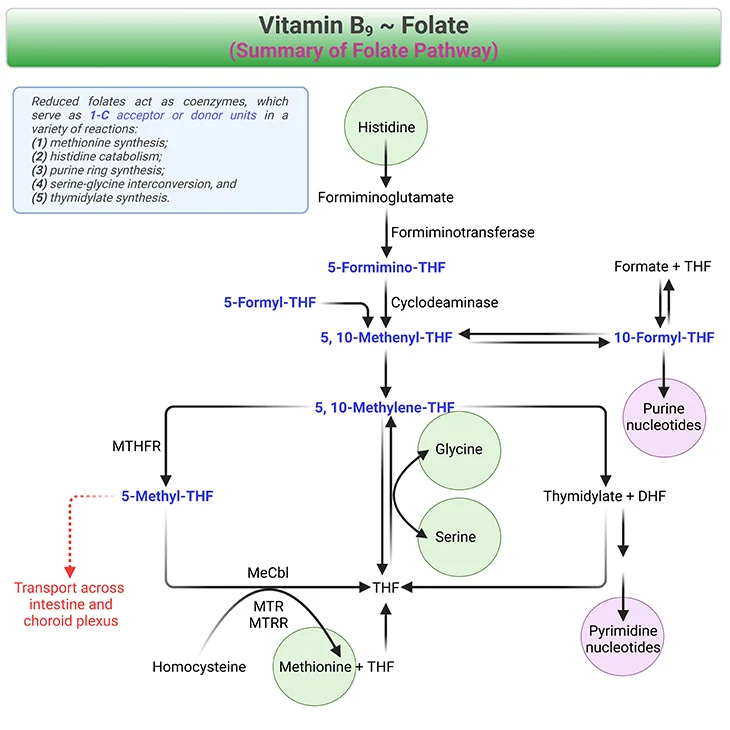
Figure 3. Summary of Folate Pathway. Reduced folates (shown in blue fonts) act as coenzymes, which serve as 1-C acceptor or donor units in a variety of reactions, viz., one carbon metabolism. For example, (1) methionine synthesis, (2) histidine catabolism, (3) purine ring synthesis, (4) serine-glycine interconversion, and (5) thymidylate synthesis. [MTHFR, methylenetetrahydrofolate reductase; MeCbl, methylcobalamin; MTR, methionine synthase; MTRR, methionine synthase reductase; THF, tetrahydrofolate]
The Methyl-Folate Trap
Cbl deficiency causes a functional folate deficiency. The methionine synthase (MS/MTR) is inactive in Cbl deficiency or after exposure to nitrous oxide. Because of the irreversible reaction of the enzyme MTHFR folate is ‘trapped’ as 5-methyl-THF and cannot be converted to THF via MS (see Figure 3) [4]. 5-methyl-THF is a poor substrate for the folylpoly-γ-glutamate synthase (FPGS), which is responsible for the addition of glutamate moieties to folates. The polyglutamate synthesis ceases, which limits the pool of intracellular polyglutamates. The de novo synthesis of purines and thymidylates grinds to a halt!
Take Home Messages
- Folate is the generic descriptor for folic acid (pteroylmonoglutamic acid) and related compounds exhibiting qualitatively the biological activity of folic acid. The term ‘folates’ refers to the compounds in this group, including mono- and polyglutamates.
- Folates are active as coenzymes in single-carbon (1-C) metabolism.
- Deficiencies of folate are manifested as anemia and dermatologic lesions.
Summary and Conclusions
In this blog, we have presented a brief overview about the structure and relationship of folic acid and its derivatives. As well as the five major metabolic functions of folate pertaining to human cells.
In summary, vitamin B9, folate, and folic acid are generic terms for a family of related compounds (vitamers) that function as coenzymes in the processing of one-carbon (1-C) units. They are derived from pteroic acid, to which one or more molecules of glutamic acid are attached. Pteroic acid is composed of a pteridine ring (2-NH2-4-OH pteridine) joined to a p-aminobenzoic acid residue.
When pteroic acid is conjugated with one molecule of L-glutamic acid, pteroylglutamic acid is formed; this can be reduced to DHF with hydrogens in positions 7 and 8, or to THF with hydrogens in positions 5, 6, 7, and 8. Only the reduced forms are biologically active. Other folate derivatives have multiple glutamic acid residues ranging from 1 to 7.
In a nutshell, folate is converted by specific enzymes to DHF, which is converted to THF and is in turn converted to methylene-THF and finally to 5-methyl-THF.
For information on autism monitoring, screening and testing please read our blog.
References
- Mitchell HK, Snell EE, Williams RJ. Journal of the American Chemical Society, Vol. 63, 1941: The concentration of “folic acid” by Herschel K. Mitchell, Esmond E. Snell, and Roger J. Williams. Nutr Rev. 1988 Sep;46(9):324-5. doi: 10.1111/j.1753-4887.1988.tb05473.x. PMID: 3067148.
https://pubmed.ncbi.nlm.nih.gov/3067148/ - Zheng Y, Cantley LC. Toward a better understanding of folate metabolism in health and disease. J Exp Med. 2019 Feb 4;216(2):253-266. doi: 10.1084/jem.20181965. Epub 2018 Dec 26. PMID: 30587505; PMCID: PMC6363433.
https://pubmed.ncbi.nlm.nih.gov/30587505/ - Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017 Jan 10;25(1):27-42. doi: 10.1016/j.cmet.2016.08.009. Epub 2016 Sep 15. PMID: 27641100; PMCID: PMC5353360.
https://pubmed.ncbi.nlm.nih.gov/27641100/ - Froese DS, Fowler B, Baumgartner MR. Vitamin B12 , folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019 Jul;42(4):673-685. doi: 10.1002/jimd.12009. Epub 2019 Jan 28. PMID: 30693532.
https://pubmed.ncbi.nlm.nih.gov/30693532/