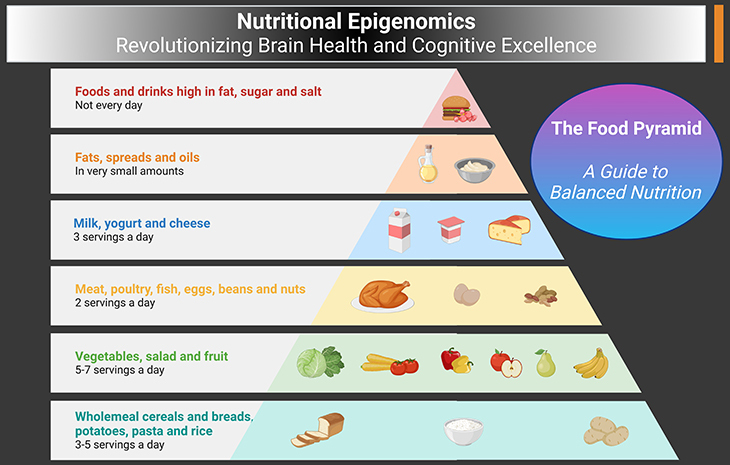
Figure 1. The Food Pyramid – A Guide to Balanced Nutrition. This figure illustrates the food pyramid, a visual representation designed to help individuals understand the importance of consuming a variety of foods for a balanced diet. (1) Base Layer – Grains and Cereals: The broad base of the pyramid represents grains and cereals such as bread, rice, pasta, and whole grains. This layer suggests that these foods should form the foundation of our daily diet, providing essential carbohydrates for energy. (2) Second Layer – Fruits and Vegetables: Above the grains, the next layer consists of fruits and vegetables. These foods are rich in vitamins, minerals, fiber, and antioxidants, contributing to overall health and well-being. It is recommended to consume a variety of colors and types to ensure a broad spectrum of nutrients (i.e., rainbow colors of fruits and vegetables). (3) Third Layer – Protein Sources: The middle section includes protein-rich foods like meat, poultry, fish, eggs, beans, nuts, and seeds. Proteins are crucial for body repair, growth, and maintenance. This layer emphasizes incorporating a mix of animal and plant-based proteins in our diet. (4) Fourth Layer – Dairy and Alternatives: This layer consists of milk, yogurt, cheese, and other dairy products or their alternatives like soy milk. These provide important nutrients such as calcium and vitamin D, essential for bone health. (5) Fifth Layer – Fats and Oils: Healthy fats and oils, such as those from avocados, nuts, seeds, and fish, occupy this tier. These fats are necessary for brain health and hormone production. However, they should be consumed in moderation. (6) Top Layer – Sugars and Sweets: At the peak of the pyramid are sugary treats and sweets. These foods should be eaten sparingly as they provide little nutritional value and are often high in calories, contributing to weight gain and other health issues – the so called “empty calories.” Balanced Diet: The pyramid emphasizes the importance of consuming larger quantities of foods from the base layers and smaller amounts from the top layers. This balanced approach ensures that individuals receive the necessary nutrients while maintaining a healthy weight and reducing the risk of chronic diseases.
Introduction
Imagine a world where your diet could unlock your brain’s full potential, where the food on your plate is not just fueling your body but also fine-tuning your mental prowess. This is the promise of nutritional epigenomics, an exciting frontier in science that is revolutionizing our understanding of brain health and cognitive function.
The Dawn of a New Era – In recent years, groundbreaking research has revealed that nutrients do far more than provide energy—they can profoundly influence our genetic makeup. Nutritional epigenomics examines how specific nutrients and dietary patterns can modify gene expression without altering the underlying DNA sequence. These modifications affect how genes are turned on or off, influencing everything from mental health to cognitive performance [1].
The Power of Nutrition – The foods we eat supply the building blocks and catalysts for complex biological processes. Vitamins, minerals, polyphenols, and other bioactive compounds can interact with our DNA and epigenetic machinery. For example, folate and vitamin B12 are vital for DNA methylation, a key epigenetic mechanism; antioxidants like curcumin and resveratrol modulate histone acetylation, impacting gene accessibility.
Cognitive Triumphs Through Diet – This field is not just theory—it has real-world implications. Diets rich in specific nutrients can enhance brain plasticity , improve learning and memory, and even mitigate the risk of neurodegenerative diseases. Omega-3 fatty acids, abundant in fish, support brain health by modulating inflammatory responses and promoting synaptic function. Green tea polyphenols, known for their epigenetic properties, offer neuroprotective benefits [2] (see Figure 1).
Balancing Act for Mental Wellness – Mental health is intricately linked to our diet. Nutritional epigenomics provides insights into how certain nutrients can benefit conditions like depression, anxiety, and schizophrenia. By modifying the epigenetic marks, nutrient therapies aim to normalize brain chemistry with minimal side effects compared to traditional pharmacological treatments.
Future Horizons – As we delve deeper into nutritional epigenomics, the potential for personalized nutrition becomes evident. The future holds the promise of tailored diets that optimize individual genetic profiles, opening up new possibilities for achieving mental and cognitive excellence.
Join us on this journey as we explore the transformative power of nutritional epigenomics, shedding light on how what we eat today can shape our brain health for tomorrow. Welcome to a revolution in brain health and cognitive excellence.
Epigenetics and Brain Functioning
Optimal mental functioning relies on well-regulated synaptic activity at neurotransmitter receptors like serotonin, dopamine, and norepinephrine. This activity depends on:
- The production levels of neurotransmitters in the brain.
- Loss of neurotransmitters through diffusion or chemical reactions.
- Presence of transporters that reabsorb neurotransmitters back into brain cells (reuptake).
Research highlights that synaptic activity is regulated by transporter concentration. Selective Serotonin Reuptake Inhibitors (SSRIs), such as Prozac and Zoloft, deactivate transporters to increase serotonin levels in synapses, while Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) like Effexor heighten both serotonin and norepinephrine activity. Conversely, benzodiazepines like Xanax and Valium enhance GABA activity by binding directly to GABA receptors, decreasing the impact of excessive norepinephrine. These medications offer substantial benefits for depression and anxiety but come with risks of addiction and side effects, including fatigue, libido loss, weight gain, and headaches [3].
Epigenetics has pinpointed nutrients such as methionine, SAMe, folic acid, niacinamide, and zinc that significantly affect neurotransmitter transporters at synapses. This correlation suggests a robust connection between nutrient therapy and recovery from mental health disorders like schizophrenia, depression, anxiety, ADHD, and behavioral issues. Further research has identified additional nutrients with the potential to enhance brain function. Nutrient therapy, aiming to normalize brain chemistry, presents the advantage of minimal side effects.
Types of Epigenetic Disorders
Epigenetic disorders are categorized into:
- Fetal programming errors.
- Deviant gene bookmarks formed later in life.
Environmental insults can lead to these deviant marks, which may persist throughout life. Fetal programming errors result in developmental disorders evident from birth, yet these errors can also predispose individuals to conditions such as cancer, heart disease, and regressive autism. Brain structure abnormalities from early epigenetic disorders tend to be irreversible, whereas brain chemistry imbalances from deviant epigenetic marks are often reversible. Future epigenetic treatments may offer lasting cures for anxiety, depression, schizophrenia, and other mental disorders [4].
Types of Epigenetic Therapies
Current epigenetic treatments focus on temporarily modifying gene expression without altering the underlying markers [5 – 6]. This is commonly achieved through either:
- Uncoiling DNA from histones to increase gene expression rates.
- Tightening DNA and histones to reduce expression rates.
These interventions improve mental function, but their benefits can wane once the treatments are halted (see Figure 2). However, emerging therapies aim to permanently correct deviant marks on DNA, potentially offering enduring solutions for mental illnesses, for example, CRSPR-based epigenome editing (see Figure 3) [7 – 8]. Developing advanced epigenetic treatments is crucial and should be given high national priority, with the goal of delivering lasting cures within the next decade or two.
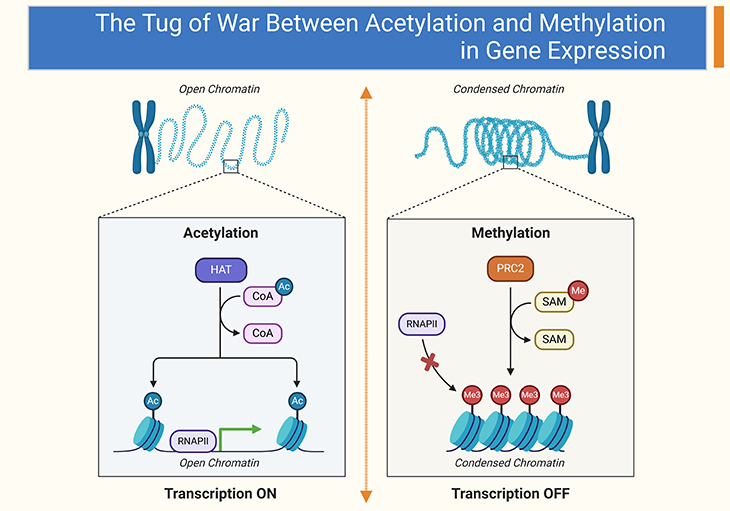
Figure 2. The Methyl-Acetyl Competition. Methyl groups are delivered to histones by SAMe (S-adenosylmethionine) (right panel), and acetyl groups by acetyl coenzyme A (left panel). Both of these chemical factors are present in high concentrations throughout the entire body. SAMe is a natural compound produced in the liver from dietary methionine. It provides methyl groups for numerous vital biochemical reactions in the body and is conserved through a process known as the ‘methylation cycle’ or ‘one-carbon cycle.’ Acetyl coenzyme A is produced from the metabolism of proteins, fats, and carbohydrates, delivering high-energy acetyl groups to the mitochondria for processing in the citric acid cycle. Both acetyl and methyl groups are essential for life. The attachment or removal of methyl and acetyl groups at histone tails is regulated by enzymes called methylases, acetylases, demethylases, and deacetylases, not by the amounts of methyl and acetyl present. Significant research aims to develop drugs that can regulate the relative amounts of these enzymes. However, certain nutrients can strongly affect these enzymes, and epigenetic nutrient therapies might be equally effective. For example, niacinamide (vitamin B3) reduces the activity of sirtuin, an important deacetylase enzyme. Similarly, folic acid influences methyl levels at histones, increasing methyl levels in tissues and the bloodstream but reducing methylation at certain histones that regulate gene expression. It is evident that many nutrients have a powerful impact on gene expression, and epigenetic nutrient therapy holds great promise.
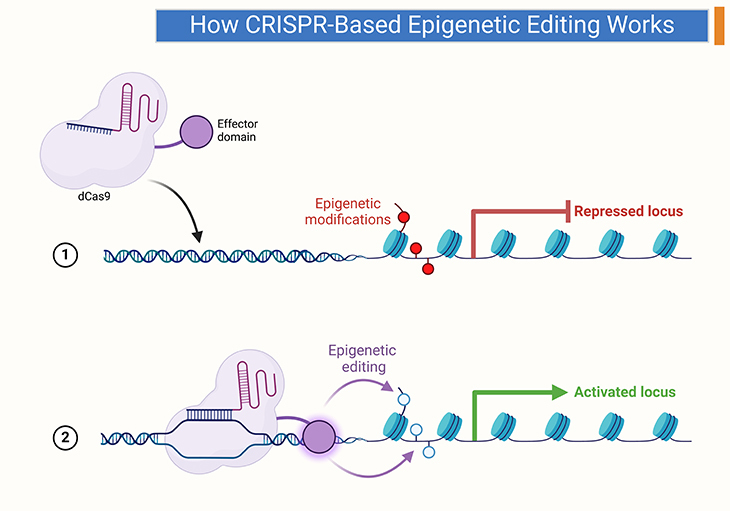
Figure 3. How CRISPR-based Epigenetic Editing Works. This figure illustrates the process of CRISPR-based epigenetic editing, a revolutionary technique used to modify gene expression. (1) Targeting DNA Sequences: The CRISPR system uses a guide RNA (gRNA) designed to match a specific DNA sequence in the genome. This gRNA directs the CRISPR protein to the exact location on the DNA that needs editing. (2) DNA Binding: Once the CRISPR protein (such as Cas9) is guided to the target DNA sequence, it binds to this specific site. (3) Epigenetic Modification: Instead of cutting the DNA like in traditional CRISPR gene-editing, CRISPR-based epigenetic editing involves adding or removing epigenetic marks, such as methyl groups. These modifications alter how tightly or loosely the DNA is coiled around histones, impacting gene expression without changing the DNA sequence itself. (4) Resulting Gene Expression Changes: By adding or removing these epigenetic marks, the treated gene can be turned on or off, leading to increased or decreased gene activity. This process allows for precise control over gene expression, offering potential therapeutic benefits for mental health and other conditions. This innovative approach aims to provide lasting changes in gene expression, with potential applications in treating various diseases by correcting abnormalities at the epigenetic level.
Epigenetics and Nutrient Therapy
Methionine and SAMe: These nutrients increase histone methylation, inhibiting gene expression of serotonin transport proteins, resulting in higher serotonin activity. They act as natural serotonin reuptake inhibitors, enhancing synaptic serotonin levels.
Folic Acid: Folic acid influences histone and DNA site methylation, acting as a serotonin reuptake enhancer. It can increase or decrease methylation based on the DNA strand portion involved. This means folic acid supplements should be tailored to patients’ methylation status.
Vitamin B3 (Niacin): Niacinamide, the active form of niacin, inhibits sirtuins, which results in higher gene expression of transporters and reduced dopamine activity. This is particularly beneficial for those with excessive dopamine levels.
Other Nutrients: Nutrients like biotin, phosphates, zinc, pantothenic acid, tryptophan, choline, and dimethylaminoethanol (DMAE) also influence brain chemistry. These nutrients impact gene expression, acetylation of histones, and enzyme function, playing crucial roles in neurotransmission and mental health.
Take-Home Messages
- Nutritional epigenomics unlocks the transformative power of diet on our brain health and mental well-being. By understanding how specific nutrients and dietary patterns can influence gene expression, we can harness these insights to enhance cognitive function, improve mental health, and reduce the risk of neurodegenerative diseases. Personalized nutrition, grounded in epigenetic principles, offers a promising path to achieving brain health and cognitive excellence.
- Invest in your future by making mindful dietary choices today—your brain will thank you!
(Cf. previous blogs entitled as: “Developmental Origins of Health and Disease: Epigenetics, Nutrition, and Infant Health.” “Generational Epigenetics: How Nutrition and Environment Shape Lifelong Brain Development.”)
Summary
Nutritional epigenomics is an evolving field that explores how the nutrients we consume can influence gene expression and, consequently, brain health and mental well-being. The central focus lies in understanding the epigenetic mechanisms—such as DNA methylation and histone modification—that are impacted by dietary components.
Key Nutrients and Their Roles:
- Methionine and SAMe: Act as natural serotonin reuptake inhibitors by increasing histone methylation, thereby enhancing serotonin activity.
- Folic Acid: Influences DNA methylation and histone modification, acting as a serotonin reuptake enhancer, with effects dependent on one’s methylation status.
- Vitamin B3 (Niacin): Niacinamide inhibits sirtuins, increasing the gene expression of transporters that reduce dopamine activity.
- Zinc, Biotin, and Polyphenols: Modulate gene expression and contribute to the regulation of neurotransmission through various epigenetic processes.
These nutrients can offer immense potential when incorporated into treatments, presenting alternatives that are both effective and minimally invasive compared to traditional pharmacological approaches.
Types of Epigenetic Therapies:
- Short-term therapies: Involves transient modifications like HDAC inhibitors and DNA methylation inhibitors, which provide temporary relief from symptoms.
- Long-term therapies: Aim to permanently modify gene expression through approaches such as CRISPR-based epigenetic editing and the use of sirtuin inhibitors. These long-lasting interventions hold the promise of addressing mental health issues at the root cause.
Conclusions
The integration of nutritional epigenomics into mental health treatment strategies marks a significant advancement in personalized medicine. By targeting specific nutritional deficiencies or needs, it is possible to modulate gene expression in a way that promotes better brain health and mitigates mental health disorders.
Advantages of Nutritional Epigenomics:
- Minimal Side Effects: Nutrient-based therapies tend to have fewer adverse side effects compared to conventional medications.
- Personalization: Treatments can be tailored based on individual genetic profiles and epigenetic marks, leading to more effective outcomes.
- Sustainable Benefits: Long-term epigenetic therapies have the potential to offer lasting improvements by correcting underlying genetic markers permanently.
Future Directions:
- Research and Development: Continued exploration into the epigenetic impacts of various nutrients and dietary patterns will enhance our ability to develop targeted therapies.
- Public Health Priority: The advancement of epigenetic therapies should be prioritized on a national level to harness their full potential in improving mental health outcomes.
In conclusion, nutritional epigenomics offers a promising pathway to revolutionize brain health and cognitive function, providing a more natural and holistic approach to mental wellness. By leveraging the interplay between diet and gene expression, we stand on the cusp of significant breakthroughs in achieving mental balance and cognitive excellence.
For information on autism monitoring, screening and testing please read our blog.
References
- Lundstrom K. Epigenetics, Nutrition, Disease and Drug Development. Curr Drug Discov Technol. 2019;16(4):386-391. doi: 10.2174/1570163815666180419154954. PMID: 29692252.
https://oap-lifescience.org/article/140/140-OAP-JCRHAP-IssuePDF.pdf - Bekdash RA. Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection. Int J Mol Sci. 2023 Jan 25;24(3):2346. doi: 10.3390/ijms24032346. PMID: 36768667; PMCID: PMC9917111.
https://pubmed.ncbi.nlm.nih.gov/36768667/ - Feehley T, O’Donnell CW, Mendlein J, Karande M, McCauley T. Drugging the epigenome in the age of precision medicine. Clin Epigenetics. 2023 Jan 11;15(1):6. doi: 10.1186/s13148-022-01419-z. PMID: 36631803; PMCID: PMC9832256.
https://pubmed.ncbi.nlm.nih.gov/36631803/
- Attig L, Gabory A, Junien C. Nutritional developmental epigenomics: immediate and long-lasting effects. Proc Nutr Soc. 2010 May;69(2):221-31. doi: 10.1017/S002966511000008X. Epub 2010 Mar 5. PMID: 20202279.
https://pubmed.ncbi.nlm.nih.gov/20202279/
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011 Mar;21(3):381-95. doi: 10.1038/cr.2011.22. Epub 2011 Feb 15. PMID: 21321607; PMCID: PMC3193420.
https://pubmed.ncbi.nlm.nih.gov/21321607/ - Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012 Aug;23(8):853-9. doi: 10.1016/j.jnutbio.2012.03.003. Epub 2012 Jun 27. PMID: 22749138; PMCID: PMC3405985.
https://pubmed.ncbi.nlm.nih.gov/22749138/ - Shi L, Li S, Zhu R, Lu C, Xu X, Li C, Huang X, Zhao X, Mao F, Li K. CRISPRepi: a multi-omic atlas for CRISPR-based epigenome editing. Nucleic Acids Res. 2024 Nov 12:gkae1039. doi: 10.1093/nar/gkae1039. Epub ahead of print. PMID: 39530233.
https://pubmed.ncbi.nlm.nih.gov/39530233/ - Reichard J, Zimmer-Bensch G. The Epigenome in Neurodevelopmental Disorders. Front Neurosci. 2021 Nov 3;15:776809. doi: 10.3389/fnins.2021.776809. PMID: 34803599; PMCID: PMC8595945.
https://pubmed.ncbi.nlm.nih.gov/34803599/