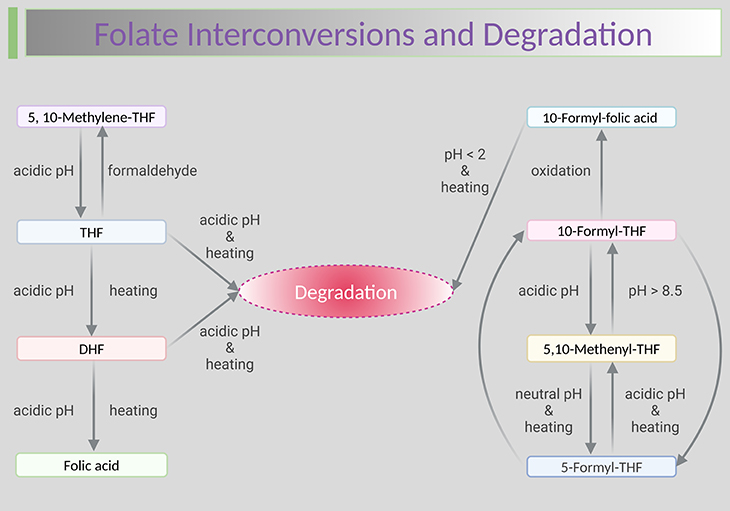
Figure 1. Folate interconversions and degradation. Stability of aqueous folic acid and folate solutions at different pH and temperature. Shown are the degradation and interconversion reactions of the folate coenzymes. Folate is the least stable of the B vitamins; careful sample handing and use of antioxidants are required to maintain sample integrity. Most importantly, a relatively small number of foods are concentrated sources of naturally occurring folate (i.e., food folate); therefore, sufficient care must be exercised while cooking. Because substantial losses of this vitamin can occur during the cooking process as a result of the combination of thermal degradation and leaching into the cooking water. [FA, folic acid; DHF, dihydrofolate; THF, tetrahydrofolate] Adapted with modifications from de Brouwer et al., 2007 [1].
Introduction
Folates are susceptible to interconversions and oxidative degradation, and their oxidation and breakdown products can also be found in serum. Some of these products no longer exhibit folate activity, nevertheless one needs to ensure that they do not interfere with the measurement of folate forms (vitamers). This means that understanding folate interconversions and degradations helps to better understand what different methods measure [1] (see Figure 1).
Measurement of Folate Vitamers in Serum, WB, and CSF
The accurate measurement of folate forms is essential for monitoring the folate intake and the evaluation of the folate status. The quantification of folate forms in whole blood (WB) reflects the long-term folate status in the body. The assay of cerebrospinal fluid (CSF) folate may be essential tool in diagnosing the cerebral folate deficiency (CFD) (cf. previous blog entitled as: ‘Folate in Health and Disease: Folate Concentrations in Serum, Whole Blood, and Cerebrospinal Fluid.’).
The quantification of folate forms from various sample materials is challenging. Most methods, including the microbiological and the protein-binding assay only determine the total folate and are unable to distinguish between the different folate forms. Several analytical difficulties delayed the development of methods for measuring the main folate forms. Examples of these factors:
- large number of folates (i.e., according to oxidation states and number of glutamate residues),
- the complex sample matrix,
- the instability and interconversions of folates, and
- the presence of folate binding proteins (FBPs) in the samples.
Folates are easier to determine in serum and where they are present as monoglutamates. In WB, folylpolyglutamates must be deconjugated to their monoglutamate forms before they can be measured. The release of folates in WB should be performed under strict temperature, pH, and time conditions, otherwise, hemolysis and deconjugation can be incomplete, or folates can be trapped in the hemoglobin molecules.
Pre-analytical Phase
Stability of folate forms in vitro
Reduced folate forms are known to be sensitive to the following [2], for instance:
- pH,
- heat,
- oxidation,
- pressure, and
- ultraviolet light (UV).
Therefore, samples preparation and measurements must be performed under controlled conditions. The major degradation products are (a) p-aminobenzoyl-L-glutamic acid (pABG) and (b) pteridine fragments that have no coenzyme function.
Tetrahydrofolate (THF), dihydrofolate (DHF), 10-formyl-THF readily undergo oxidative degradation in vitro. Moreover, reduced folates undergo pH-dependent enzymatic and non-enzymatic interconversions. The pH and heat stability of individual folates forms in in vitro experiments are summarized in Figure 1.
Folic acid (FA) and 5-methyl-THF are stable at pH 2–10 and 5-formyl-THF is relatively stable at 37◦C and pH 3–10. THF is relatively stable below pH < 5, whereas DHF is relatively stable at pH > 8. In contrast to THF, DHF is instable under all pH conditions after heating. Under low pH conditions, THF can be oxidized in vitro to DHF and FA (see Figure 1).
In order to stabilize the reduced folates during sample preparation, it is decisive to add adequate antioxidant such as ascorbic acid, β-mercaptoethanol, and dithiothreitol (DTT). In addition, artificial FA might be generated from the oxidation of THF during sample preparation, even in the presence of antioxidants. This finding might lead to misinterpretation of studies looking at unmetabolized FA in the samples.
Analytical Phase
Quantification methods
1. Microbiological assay (gold standard)
The microbiological assay (MBA) was introduced over 50 years ago. This assay is based on the fact that a specific microorganism, for example, Lactobacillus rhamnosus, formerly known as Lactobacillus casei, grows proportionally to the available folate in the sample [3].
The MBA has received renewed interest during the past decade because of improvements in efficiency and robustness, so that the assay can be reliably used in a high-throughput routine setting such as national health and nutrition examination survey (NHANES) [4]. Important improvements introduced in the 1970s and 1980s included, for example:
- the development of a chloramphenicol-resistant strain of L. rhamnosus,
- the ability to cryopreserve the inoculum, and
- the introduction of automated microtiter plate technology.
2. Protein-binding assays
Protein-binding assays (PBAs) were developed with the clinical laboratory in mind, to enable the diagnosis of folate deficiency. These assays use the highly specific folate binding protein (FBP) (mainly from milk or milk fractions, sometimes from porcine plasma or kidney) to ‘extract’ folate from the sample [5].
The protein-binding assays represent the next assay generation. One example is the automated chemiluminescent immunoassay on the ADVIA Centaur platform (ADVIA Centaur XP System, Siemens Healthcare Diagnostics GMbH, Eschborn, Germany). This method consists of a competitive assay using FBP and depends on the release of the folate from its binding proteins. Before folate can be measured, samples are treated with DTT to release the folate from endogenous binding proteins. The released folate competes against acridinium ester-labeled FA for a limited amount of biotin-labeled FBP. The biotin-labeled FBP binds to avidin, which is covalently bound to paramagnetic particles in the solid phase. The amount of sample folate correlates inversely with the obtained signal.
3. Chromatography-based assays
Methods used for quantifying total folate show large disagreements and are unable to detect various forms of the vitamins. In the 1970s and 1980s, first chromatographic methods have been developed. These methods can distinguish between the different folate forms. Numerous high performance liquid chromatography (HPLC) and gas chromatography (GC) methods have been described for the detection of folate forms in serum and WB (see Figure 2). Recently, liquid chromatography coupled with mass spectrometry (LC-MS) methods and liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods have been developed to quantify folate monoglutamates in biological fluids and foods. LC-MS/MS methods provide higher levels of selectivity (see Figure 2).
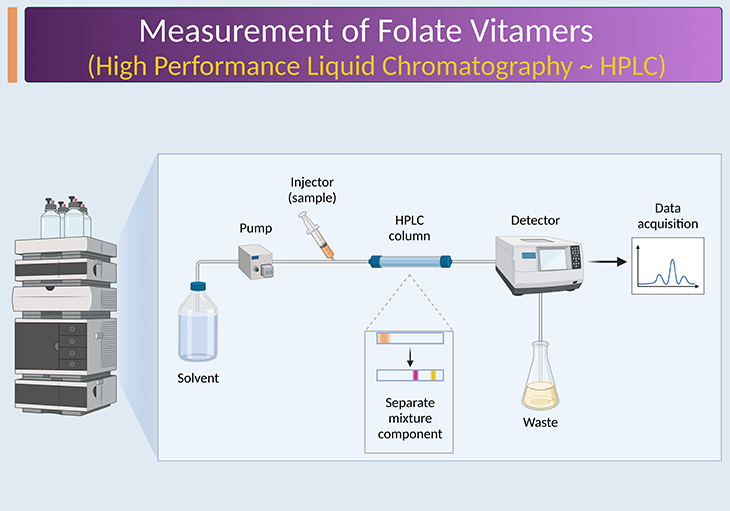
Figure 2. Chromatography-based assays. Chromatography based methods provide information on individual folate forms and are based on either measurement of intact folates via high performance liquid chromatography (HPLC) or measurement of folate breakdown products as an indicator for total folate via gas chromatography (GC). Over the past decade, tandem mass spectrometers (MS/MS) have become more affordable and user-friendly and smaller in footprint, and their performance in terms of sensitivity and linear dynamic range has greatly improved. They are now considered to be the preferred detectors for HPLC-based methods, i.e., liquid chromatography with tandem mass spectrometry (LC-MS/MS), at least for specialized laboratories.
Modern LC-MS/MS methods depend on sample extraction and clean-up and are high sensitive in the nanomolar range. Monoglutamate or polyglutamate folate standards and stable isotope-labeled internal standards for most of the monoglutamate forms are available. However, at present it is still not possible to detect all available folate forms with one method.
Recently an ultra-performance LC-MS/MS method for the fast, sensitive, and reliable determination of key folate forms (5-methyl-THF, 5-formyl-THF, 5,10-methenyl-THF, THF, and FA) in serum and of 5-methyl-THF and non-methyl-THF (sum of formyl-THF, 5,10-methenyl-THF, 5,10-methylene-THF, THF, DHF, and FA) has been described.
For example, the methods in serum shows strong correlation with the immunological method on the ADVIA Centaur platform (R = 0.939, p < 0.001). Despite the good correlation, the results for the quantification of the folates may vary greatly in individuals due to the non-specific detection of the folate forms via the immunological methods. The ultra-performance LC-MS/MS methods are preferred over the immunological or microbiological methods when accurate information about the folate forms distribution is needed [6].
Take Home Messages
Serum folate
- Serum folate represents the sum of several folate vitamers (forms) circulating in the blood stream often referred to as ‘total folate.’
- The analysis of these folate compounds is complicated by their appearance as (i) pteroylmonoglutamates with variations in the oxidation state of the pteridine moiety and (ii) in the one-carbon substituent group at the N-5 and/or N-10 positions.
- The main circulating folate vitamer is 5-methyl-THF.
- Unmetabolized folic acid can be present in varying concentrations.
- Reduced folate vitamers such as THF and formyl-folates (viz., 5- or 10-formyl-THF sometimes 5,10-methenyl-THF) may also be present, albeit in very small concentrations.
RBC folate
- RBCs contain much higher folate concentrations than serum.
- 5-Methyl-THF polyglutamates are the main folate forms.
- In persons with the MTHFR 677 C>T polymorphism, a portion of the 5-methyl-THF polyglutamates is replaced by formyl-folates.
- The measurement of RBC folate is even more complex than that of serum folate, because of the need to convert polyglutamates to monoglutamates before analysis.
Main analytical method types used for the measurement of serum and RBC folate
- Microbiological assay for total folate (gold standard).
- Protein-binding assay for total folate.
- Chromatography-based MS/MS (tandem mass spectrometry) assay for folate vitamers.
Summary and Conclusions
In this blog we have presented a succinct overview concerning the stability of various folate forms that are to be considered during the preanalytical process. In addition, we have briefly highlighted on how to determine various forms of folate in serum, WB, and CSF using various analytical techniques that are currently employed by most of the clinical laboratories, as of today; and their advantages and disadvantages.
First, The microbiological method is the earliest method to determine folate in various biological samples. Over a period of time it was not used as frequently, surpassed by more user-friendly protein-binding assays and more specific and selective chromatographic assays. In general, once newer methods become established and recognized, they tend to make older methods obsolete. Interestingly, for the microbiological assay (being the gold standard) this does not seem to be the case.
Second, the emergence of isotope-dilution/liquid chromatography/tandem mass spectrometry (ID/LC/MS/MS) methods has provided the folate field for the first time with higher-order reference methods. The fact that these methods shown comparatively good agreement with the microbiological assay reinforces the validity of the latter. The microbiological assay will not be the method of choice for highly specialized clinical laboratories that are keen on more than total folate. However, this assay can and potentially will be the method of choice for low-resource settings.
Next, most clinical laboratories use protein-binding assays because these are currently the only methods that provide the high-throughput coupled with a relative low cost, and they do not require extensive technical expertise. This is not about to change in the near future. It is expected that manufacturers will become more engaged in normalizing their assays to reference materials that have been characterized by ID/LC/MS/MS and that more of these reference materials will become available.
Finally, HPLC-based methods can provide considerable detail when one is determining folate patterns in biological samples. Nevertheless, because of the lack of suitable internal standards (ISs), they have to be conducted with utmost care. The greatest developmental changes in recent years have been associated with MS-based methods that have contributed significantly to anchoring folate measurements, facilitating a better understanding of method differences. Moreover, with increases in automation and decreases in cost, their role will continually grow.
For information on autism monitoring, screening and testing please read our blog.
References
- De Brouwer V, Zhang GF, Storozhenko S, Straeten DV, Lambert WE. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem Anal. 2007 Nov-Dec;18(6):496-508. doi: 10.1002/pca.1006. PMID: 17624887.
https://pubmed.ncbi.nlm.nih.gov/17624887/ - Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, Molloy AM, Caudill MA, Shane B, Berry RJ, Bailey RL, Hausman DB, Raghavan R, Raiten DJ. Biomarkers of Nutrition for Development-Folate Review. J Nutr. 2015 Jul;145(7):1636S-1680S. doi: 10.3945/jn.114.206599. Epub 2015 Jun 3. PMID: 26451605; PMCID: PMC4478945.
https://pubmed.ncbi.nlm.nih.gov/26451605/ - Gregory JF 3rd. Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Adv Food Nutr Res. 1989;33:1-101. doi: 10.1016/s1043-4526(08)60126-6. PMID: 2697230.
https://pubmed.ncbi.nlm.nih.gov/2697230/ - Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr. 2011 Jul;94(1):322S-331S. doi: 10.3945/ajcn.111.013300. Epub 2011 May 18. PMID: 21593508; PMCID: PMC3127520.
https://pubmed.ncbi.nlm.nih.gov/21593508/ - Wilson DH, Williams G, Herrmann R, Wiesner D, Brookhart P. Issues in immunoassay standardization: the ARCHITECT Folate model for intermethod harmonization. Clin Chem. 2005 Apr;51(4):684-7. doi: 10.1373/clinchem.2004.042358. PMID: 15788785.
https://pubmed.ncbi.nlm.nih.gov/15788785/ - Hannisdal R, Ueland PM, Svardal A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin Chem. 2009 Jun;55(6):1147-54. doi: 10.1373/clinchem.2008.114389. Epub 2009 Apr 9. PMID: 19359539.
https://pubmed.ncbi.nlm.nih.gov/19359539/




