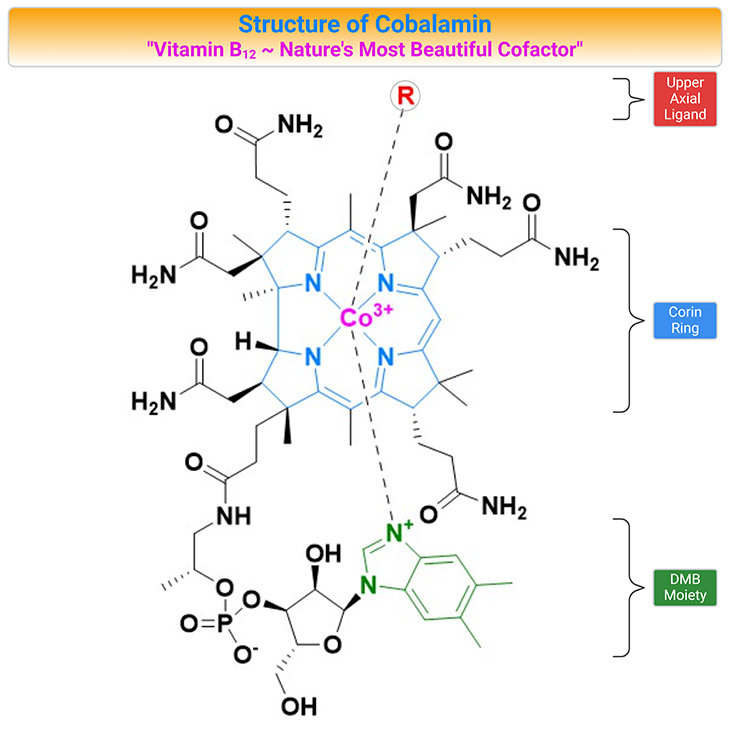
Figure 1. Structure of Cobalamin (Cbl). Cobalamin (vitamin B12) belongs to a group of compounds of similar chemical structure but with completely different biological functions. The vitamin is composed of a corrin ring surrounding a central cobalt atom that is bound to a lower (alpha-axial) and an upper (beta-axial) ligand. The lower ligand consists of a benzimidazole group (5,6-dimethylbenzimidazole, DMB) that through a ribose-phosphate group is attached to the coring ring. The upper ligand is unique for each form of the vitamin. In the co-factor or coenzyme forms, the upper ligand is either a methyl (methylcobalamin, MeCbl) or a 5’-deoxyadenosyl (adenosylcobalamin, AdoCbl) group. The synthetic forms of Cbl are cyanocobalamin (CNCbl) and hydroxycobalamin (OHCbl).
Introduction
Once called ‘nature’s most beautiful cofactor,” cobalamin (Cbl; vitamin B12) is at least nature’s most chemically complex coenzyme [1]. The molecule, vitamin B12, contains a cobalt atom, a rarity in natural products. One reason that metals are marvelous is that many of them can combine with organic structures to make organometallic compounds that are essential for life, for example, chlorophyll, in which the metal is magnesium; and cobalamin, in which the metal is cobalt (see Figure 1).
The discovery of Cbl followed intensive studies on pernicious anemia (PA), a previously fatal medical condition. In the early 1920s, Minot and Murphy demonstrated that they were able to cure PA by whole liver extracts, which were subsequently used for treatment – as oral liver extract! Later, it was shown that liver is an important source of Cbl.
Animals can obtain Cbl by consuming foods contaminated with synthesizing bacteria and then incorporate the vitamin into their body organs. As a result, foods of animal source are the only natural source of Cbl in the human diet. Cbl is synthesized exclusively in microorganisms. In humans, Cbl is an essential cofactor for methyl group transfer and cell division.
Cbl is a water-soluble vitamin from the B-group. It is essential for cell growth and division. Cbl deficiency can cause severe hematological and/or neurological manifestations. Low serum Cbl levels are associated with pregnancy loss. Inherited defects in Cbl metabolism are mostly associated with, for example:
- failure to thrive,
- irritation,
- feeding problems, and
- neurological or neurodevelopmental disorders.
Hematological and neurological symptoms of folate and Cbl deficiency are similar which is consistent with the crosstalk between the folate and Cbl pathways [2]. Therefore, this review aims to provide an overview on Cbl metabolism with a brief description of absorption, transport, and intracellular uptake, as well as metabolic pathways.
Biochemistry Cobalamin (Cbl)
Cbl belongs to a group of compounds of similar chemical structure but with completely different biological functions. Cbl is a large organometallic molecule, ≈ 13,000 – 15,000 Da in size, and is the most chemically complex vitamin known. The focal point of vitamin B12 is the central cobalt atom, which has up to six ligands bound to it.
The vitamin is composed of a corrin ring surrounding a central cobalt atom that is bound to a lower (α-axial) and an upper (β-axial) ligand (see Figure 1, 2). The lower ligand consists of a benzimidazole group (5,6-dimethylbenzimidazole, DMB) that through a ribose-phosphate group is attached to the coring ring. The upper ligand is unique for each form of the vitamin. In the co-factor or coenzyme forms, the upper ligand is either a methyl (methylcobalamin, MeCbl) or a 5’-deoxyadenosyl (adenosylcobalamin, AdoCbl) group (see Figure 2, 3).
The vitamin is crucially involved in the proliferation, maturation, and regeneration of cells. Cbl maintains low homocysteine (Hcy) levels by transferring a methyl group from 5-methyltetrahydrofolate (5-methylTHF) to Hcy converting it into methionine (Met). The synthetic forms of Cbl are cyanocobalamin (CNCbl) and hydroxycobalamin (OHCbl). OHCbl is an abundant and physiologically relevant intermediate form. Both OHCbl and CNCbl are frequently used for supplementation. MeCbl and AdoCbl have recently emerged as alternative forms in supplements (see Figure 2, 3).
There are three important, inter-related factors that influence Cbl reactivity and function, for instance:
- the oxidation state of cobalt;
- whether the DMB is coordinated to cobalt in the lower axial position (α-face); and
- the identity of the R-group bound in the upper axial position (β-face).
Additionally, the cobalt atom of cobalamin may exist in the +3 [cob(III)alamin], +2 [cob(II)alamin], or +1 [cob(I)alamin] oxidation state [3].
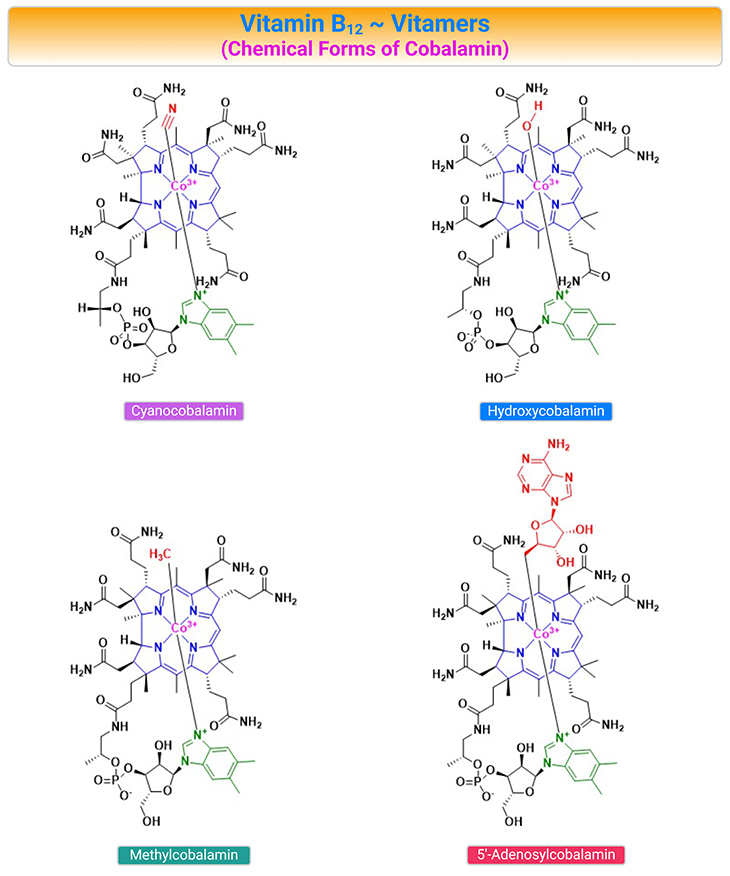
Figure 2. Chemical Structures of the B12 Vitamers. Cobalamin (Cbl) is a collective name for structurally related compounds (vitamers) that contain a central cobalt (Co) ion coordinated to four equatorial nitrogen atoms donated by the tetrapyrrolic corrin ring. The Cbl structure includes two axial positions, where different ligands can coordinate to the Co ion (usually Co3+). The ligand on the upper surface of the ring (β-face) may be cyanide (CN–), water H2O, or OH depending on the pH of the surroundings), or alkyl-group [such as methyl (Me) or 5’-deoxyadenosyl (Ado)], or other ligands. The nucleotide base, 5,6-dimethylbenzimidazole (DMB), constitutes the ligand on the lower surface of the corrin ring (α-face) in cobalamin forms.
The Co-ion in the center of the Cbl can exist in three oxidation states (Co3+, Co2+, and Co1+), which appear in a strict sequence under the catalytic transformations. The oxidation state of Cbl affects the association of the upper and lower ligands of the coring ring. Cob(I)alamin usually has no axial ligands coordinated to the Co-ion, whereas cob(II)alamin and cob(III)alamin have one and two axial positions occupied by DMB and DMB + upper ligand, respectively.
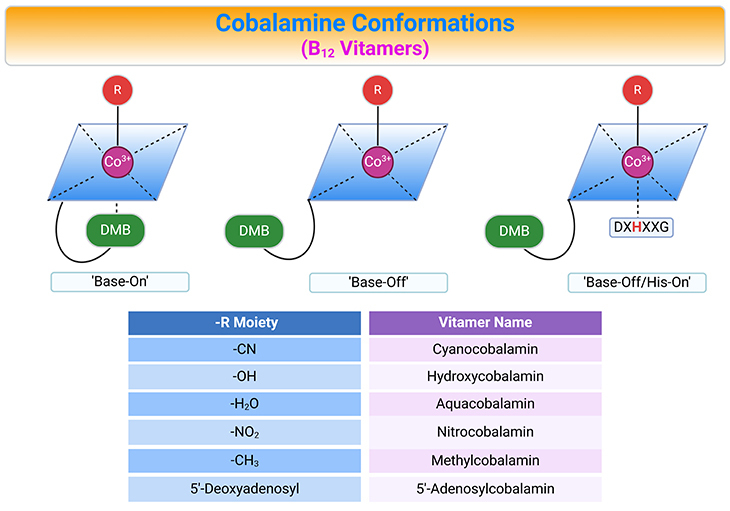
Figure 3. Cobalamin Conformations. Alternative conformations of cobalamins (Cbl) differing with respect to the lower or α-axial ligation site (top panel). The variable upper or β-axial ligands are denoted as R and corresponding B12 vitamers (bottom panel, table). AdoCbl, MeCbl, CNCbl, and OHCbl, all of which are cob(III)alamins, prefer to adopt a configuration where the DMB (5,6-dimethylbenzimidazole, DMB) nitrogen base is coordinated to the cobalt in the lower axial position (referred to as ‘base-on’ configuration). Some enzymes, however, are able to shift these cob(III)alamins to the ‘base-off’ configuration. Interestingly, enzymes such as methionine synthase (MS) and methylmalonyl CoA mutase (MCM), which use MeCbl and AdoCbl as cofactors, respectively, bind the cobalamin so that DMB nitrogen is displaced from the cobalt and replaced by a histidine of the enzyme. This type of binding is considered ‘base-off/His-on’ and is important for the catalytic activity of the enzymes.
Generally, cob(III)alamin and cob(II)alamin exist in solution in their ‘base-on’ forms, meaning that DMB is coordinated at the α-face of the corrin ring. The Co-N coordination bond between cobalt and DMB is, however, not strong, and the nucleotide base can dissociate depending on: (a) pH, (b) temperature, (c) protein environment, and (d) oxidation state of the Co-ion. Cbl-configuration with the dissociated base is called ‘base-off.’
Cobalamin transporting proteins in mammals, such as haptocorrin (HC), intrinsic factor (IF), and transcobalamin II (TC) recognize and bind the ‘base-on’ cobalamins. In contrast, Cbl-dependent MCM, MS, and chaperon MMACHC (methylmalonic aciduria and homocystinuria type C protein) bind Cbl in a ‘base-off’ form. The switch between ‘base-on’ and ‘base-off’ configuration modifies the reactivity of the Co-associated upper ligand and enables the changes in redox chemistry needed for the enzymatic activity (see Figure 1 – 3).
Metabolic Function and Transport of Cobalamin in Human Cell
Cobalamin Chemical Forms and Activation of Cbl-Dependent Enzymes
The upper ligand of Cbl has a major role in activation of the Cbl-dependent enzymes. There are only two forms of Cbl that have biological activity as cofactors in enzymatic reactions (See Figure 4) These are AdoCbl and MeCbl. In mammalian cells, Cbl is required for only two key enzymatic reactions, for instance:
- On the one hand, AdoCbl is a cofactor for L-methylmalonyl-CoA mutase (MCM) that is involved in the isomerization of L-methylmalonyl-CoA (MM-CoA) to succinyl-CoA (Succ-CoA). The excess of MM-CoA is converted into methylmalonic acid (MMA). Cbl deficiency leads to enhanced conversion of MM-CoA into MMA and elevation of the latter compound in the blood.
- On the other hand, MeCbl is a cofactor for MS that transfers a methyl group from 5-methylTHF to Hcy during the synthesis of methionine. (cf. previous blog entitled as ‘Folate in Health and Disease: Biochemistry and Function of Folates’ )
The MeCbl-mediated reaction occurs in the cytosol while the AdoCbl-mediated pathway takes place in the mitochondria (see Figure 4). In case of Cbl deficiency these reactions are inhibited and Hcy as well as MMA will increase. Therefore, concentrations of MMA and total Hcy (tHcy) in plasma are priority markers for Cbl status.
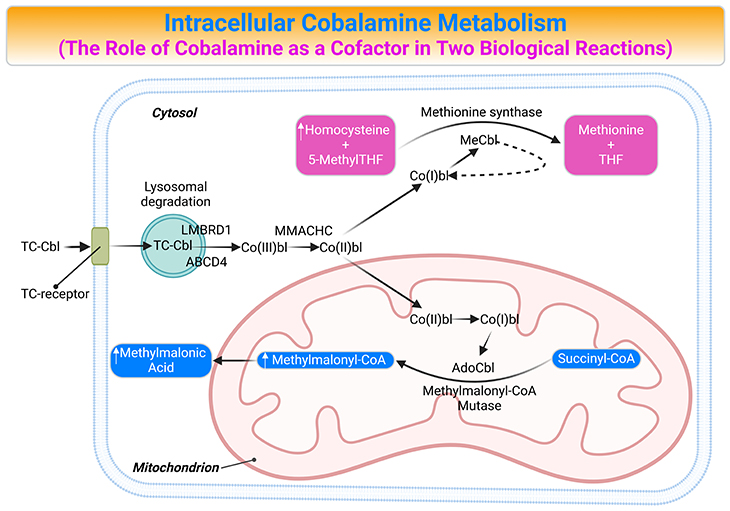
Figure 4. Intracellular Cobalamin Metabolism. The role of cobalamin (Cbl) as a cofactor in two biological reactions and the consequences of cobalamin deficiency. Trafficking and assimilation of all forms of Cbl into mammalian cells is likely to follow the same following steps: (a) crossing the intestinal barrier (absorption); (b) cellular internalization; (c) lysosomal-processing and release; (d) dealkylation (remove Ado, Me), decyanation (remove CN), or reduction (remove CN, HO); (e) forming the coenzymes; and (f) then attachment to the corresponding enzymes, i.e., the final destination methionine synthase (MS) and methylmalonyl-CoA mutase (MCM or MUT). [ABCD4, ATP-binding cassette, subfamily D, member4; AdoCbl, adenosylcobalamin; Cbl, cobalamin; Co(III)bl, cob(III)alamin; Co(II)bl, cob(II)alamin; Co(I)bl, cob(I)alamin; Hcy, homocysteine; LMBRD1, LMBR1 domain-containing protein 1; MMACHC; Metabolism of cobalamin associated C; MCM, L-methylmalonyl CoA mutase; MeCbl, Methylcobalamin; Met, Methionine; 5-MethylTHF, 5-methltetrahydrofolate; MMA, methylmalonic acid; MM-CoA, L-methylmalonyl CoA; MS, methionine synthase; Succ-CoA, succinyl CoA; TC, transcobalamin II; THF, tetrahydrofolate]
The Role of Vitamin B12 as a Cofactor and its Wide-Ranging Implications
Vitamin B12 (cobalamin, Cbl) is a micronutrient essential to human health; and it is not produced in humans but is required for the function of two enzymes: (i) cytosolic methionine synthase (MS) and (ii) mitochondrial methylmalonyl-CoA mutase (MCM or MUT).
MCM utilizes the adenosylated form of Cbl (AdoCbl) to catalyze the conversion of L-methylmalonyl-CoA to succinyl-CoA. This is an essential step in the catabolism of branched-chain amino acids, odd-chain fatty acids, and the side chain of cholesterol, and contributes to anaplerotic replenishment of the tricarboxylic acid cycle (TCA cycle). [Greek anaplerosis: (ana), up; (plero), to fill – i.e., to supply intermediary metabolites to maintain pathway integrity]
By contrast, MS requires the methylated form of Cbl (MeCbl) and catalyzes the remethylation of homocysteine to methionine using 5-methyltetrahydrofolate (5-methylTHF) as methyl donor. The importance of this reaction extends beyond the production of methionine, an essential proteinogenic amino acid, because methionine is further converted to S-adenosylmethionine (AdoMet, often called SAM), a universal methyl donor.
The methyl group of SAM can be donated to form a wide range of vitally important methylated compounds, for instance, creatine, epinephrine, and sarcosine; as well as methylated RNA, DNA, and proteins. Consequently, This utilization of 5-methylTHF as methyl donor inexorably links Cbl metabolism with folate-mediated one-carbon (1-C) metabolism. The significance of these pathways to human health is underlined by the extent and severity of disease caused by their primary and secondary dysfunction.
Cellular Trafficking of Cobalamin
Since vitamin B12 is made by just a few microorganisms, it is acquired through dietary uptake in animals. Human dietary source, such as milk, egg, fish, liver, kidney, and meat, include vitamin B12 in quantities in excess of a few micrograms a day. In humans, the absorption, transport, and cellular uptake of cobalamin is complex (see Figure 5; Table 1) [4, 5].
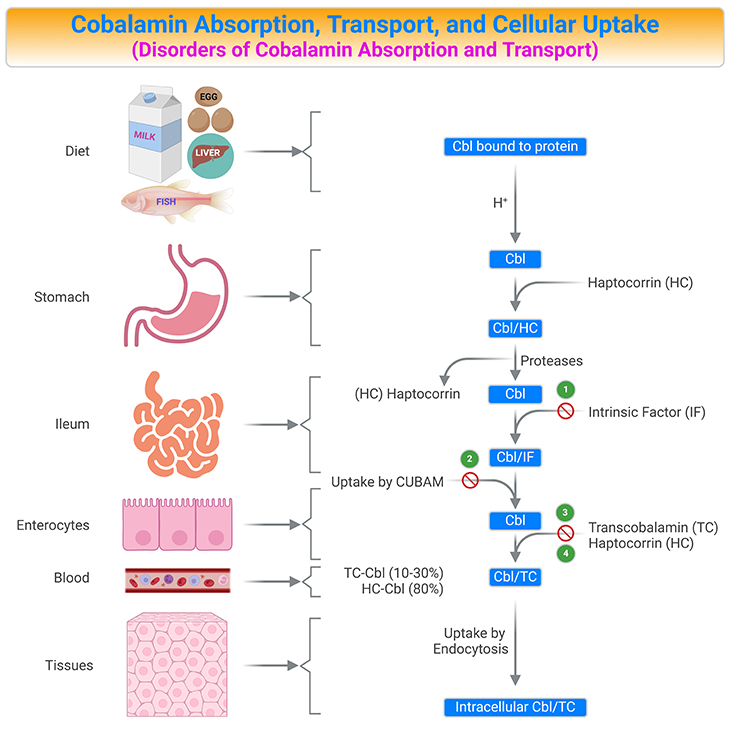
Figure 5. Summary of Cobalamin Absorption, Transport, and Cellular Uptake. Human vitamin B12 (cobalamin, Cbl) ingestion and absorption. Parietal cells of the stomach release intrinsic factor (IF). Both vitamin B12 and IF are delivered to the duodenum. In the upper small intestine, vitamin B12 binds to IF. In the terminal ileum, the vitamin B12/IF (Cbl/IF) complex is taken up into the enterocyte at this point in the small intestine. Most of the absorbed vitamin B12 is stored in the liver and the remainder is delivered to other tissues. [Cbl, cobalamin; Cbl/HC, Cbl-haptocorrin complex; Cbl/IF, Cbl-intrinsic factor complex; Cbl/TC, Cbl-transcobalamin II complex; CUBAM, ileal receptors made up of cubilin and amnionless proteins; HC, haptocorrin (transcobalamin I); TC, transcobalamin II] {(1) Intrinsic factor deficiency; (2) Imerslϋnd-Grasbeck disease; (3) Trans-Cbl deficiency; (4) Haptocorrin deficiency.}
Cbl is released from its food-binding proteins by the action of gastric juice and proteolytic enzymes. It is then captured by haptocorrin (also known as transcobalamin I), an R-binder protein in the saliva and stomach. In the upper small intestine, pancreatic enzymes and an alkaline pH degrade the haptocorrin-Cbl complex (Cbl/HC).
The released free vitamin is then captured by intrinsic factor (IF), another Cbl binding protein. Parietal cells of the stomach release IF. In the terminal ileum, the intrinsic factor-Cbl complex (Cbl/IF) binds to IF receptors (a receptor composed of a heterodimer of amnionless and cubilin, called cubam) on the apical surface of ileal enterocytes and is then transferred through the ileal membrane by endocytosis. Once inside the enterocytes, IF is degraded in the lysosomes and Cbl is released into the cytosol (LMBRD1 and ABCD4 are lysosomal membrane exporters), where it is transported across the ileal receptor cell and released into the bloodstream, possibility by the multidrug resistance protein MRP1 (see Table 1; Figure5).
In the bloodstream, Cbl binds to a third binding protein, transcobalamin II (TC) or haptocorrin (HC) [TC-Cbl ≈ 10-30%; HC-Cbl ≈ 80%]. TC is synthesized within the enterocytes and is the only binder that can deliver Cbl into cells via TC-receptor (Cbl/TC). The Cbl/TC complex, named holotranscobalamin (holoTC), is released into the portal circulation, arrives the blood circulation, and circulates until it is taken up the peripheral cells.
Cbl bound to TC (Cbl/TC) is recognized by TC-receptors (CD320) that are expressed by all cell types. A maximum of 30% of circulating Cbl is bound to TC, which represents metabolically active Cbl. The remaining part of Cbl (around 70 -80%) is bound to haptocorrin and is called holohaptocorrin (holoHC). The receptor-mediated absorption of Cbl is a saturable process and a maximal amount of 3 μg of the vitamin per meal is thought to be internalized via this pathway (see Figure 5; Table 1).
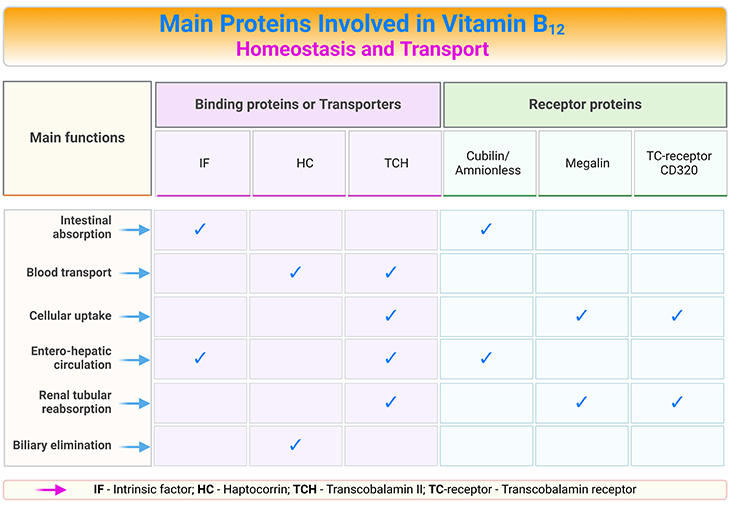
Table 1. Main Proteins Involved in Vitamin B12 Homeostasis and Transport.
A considerable amount of Cbl is secreted into the bile. Two-thirds (2/3rd) of the secreted Cbl in the bile is reabsorbed once again in the ileum. Generally, the liver contains most of the Cbl in the body (2 – 3 mg). The kidney and brain also accumulates Cbl. Cbl is excreted into urine, and this can be reabsorbed in the proximal tubules via a specific receptor (viz., megalin/cubilin). The major route by which Cbl is lost from the body is through the feces.
Take Home Messages
- Vitamin B12 is the generic descriptor for all corrinoids (compounds containing the cobalt-centered corrin nucleus) exhibiting the biological activity of cyanocobalamin.
- The chief natural sources of vitamin B12 are milk, egg, fish, liver, kidney, and meat.
- Deficiencies of vitamin B12 are manifested as anemia and neurologic changes; and can be fatal.
- There are only two forms of cobalamin that have biological activity as cofactors in enzymatic reactions. These are adenosylcobalamin and methylcobalamin.
- The synthetic forms of cobalamin are cyanocobalamin and hydroxycobalamin.
- Vitamin B12 is a micronutrient essential to human health; and it is not produced in humans but is required for the function of two enzymes, viz., cytosolic methionine synthase and mitochondrial methylmalonyl-CoA mutase.
- Vitamin B12 is also necessary to convert methylmalonyl CoA to succinyl CoA, thus facilitating its entry into the citric acid cycle. This occurs in the mitochondria.
- Tetrahydrofolate is synthesized from 5-methyltetrafolate by transferring a methyl group to homocysteine via cobalamin. In this process, methionine is formed from homocysteine by methionine synthase, a vitamin B12-dependent enzyme. This occurs in the cytosol.
- The function of vitamin B12 in single-carbon (1-C) metabolism is interrelated with that of folate.
Summary and Conclusions
In this blog, we have provided an overview of the marvelous structure of vitamin B12 and its metabolism; and a concise description of absorption and intracellular metabolic pathways. As well as the significance of crosstalk between the folate and Cbl pathways and its wide-ranging implications .
In a nutshell, vitamin B12 is a micronutrient essential to human health. Due to its complex structure and dual cofactor forms, vitamin B12 undergoes an elaborate series of absorptive and processing steps before serving as cofactor for the enzymes methylmalonyl-CoA mutase and methionine synthase. Deficiencies vitamin B12 result in problems affecting multiple systems, including hematologic, neurologic, dermatologic, ophthalmologic, cardiovascular, and renal; and can be fetal!
For information on autism monitoring, screening and testing please read our blog.
References
- Stubbe J. Binding site revealed of nature’s most beautiful cofactor. Science. 1994 Dec 9;266(5191):1663-4. doi: 10.1126/science.7992049. PMID: 7992049.
https://pubmed.ncbi.nlm.nih.gov/7992049/ - Chanarin I, Deacon R, Lumb M, Perry J. Cobalamin and folate: recent developments. J Clin Pathol. 1992 Apr;45(4):277-83. doi: 10.1136/jcp.45.4.277. PMID: 1577963; PMCID: PMC495263.
https://pubmed.ncbi.nlm.nih.gov/1577963/
- Obeid R, Fedosov SN, Nexo E. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol Nutr Food Res. 2015 Jul;59(7):1364-72. doi: 10.1002/mnfr.201500019. Epub 2015 May 12. PMID: 25820384; PMCID: PMC4692085.
https://pubmed.ncbi.nlm.nih.gov/25820384/ - Gherasim C, Lofgren M, Banerjee R. Navigating the B(12) road: assimilation, delivery, and disorders of cobalamin. J Biol Chem. 2013 May 10;288(19):13186-93. doi: 10.1074/jbc.R113.458810. Epub 2013 Mar 28. PMID: 23539619; PMCID: PMC3650358.
https://pubmed.ncbi.nlm.nih.gov/23539619/ - McCorvie TJ, Ferreira D, Yue WW, Froese DS. The complex machinery of human cobalamin metabolism. J Inherit Metab Dis. 2023 May;46(3):406-420. doi: 10.1002/jimd.12593. Epub 2023 Feb 8. PMID: 36680553.
https://pubmed.ncbi.nlm.nih.gov/36680553/