Table of Contents
- Introduction
- ATP: The Universal Energy Currency—A Story of Discovery
- The Myth of the Squiggle: Rethinking ATP’s High-Energy Bond
- ATP Production: The Enzyme Factories Behind Cellular Power
- The Biochemical Paradox and the Search for Order
- Take-Home Messages
- Summary and Conclusions
- Did You Know About Folate Receptor Autoantibodies (FRAAs) and Brain Development?
- References
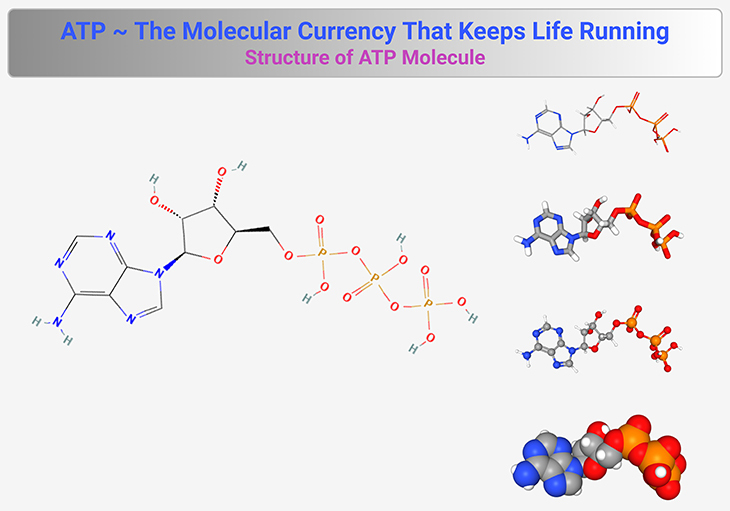
Figure 1. Structure of ATP Molecule. This figure illustrates the molecular structure of adenosine triphosphate (ATP), the universal energy carrier of life. ATP consists of three key components: (1) Adenine – A nitrogenous base that forms part of the ATP backbone. (2) Ribose – A five-carbon sugar that connects adenine to the phosphate groups. (3) Three Phosphate Groups – Linked in a chain, these phosphates store and release energy through hydrolysis. The terminal phosphate bond (often depicted with a squiggle, ~) is highly reactive and, when broken, releases energy to drive cellular processes. ATP readily converts to adenosine diphosphate (ADP) and inorganic phosphate (Pᵢ), facilitating energy transfer in metabolism. The reversible ATP ↔ ADP cycle ensures continuous energy availability for biological functions, including muscle contraction, enzyme activation, and biosynthesis. {Image credits – modified and adapted from: National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 5957, Adenosine Triphosphate. Retrieved May 11, 2025.} [https://pubchem.ncbi.nlm.nih.gov/compound/Adenosine-Triphosphate].
Introduction
Life depends on energy, and cells have evolved intricate mechanisms to harness, store, and distribute it with remarkable efficiency. At the center of this energy management system is adenosine triphosphate (ATP), widely regarded as the universal energy currency of life (see Figure 1). ATP fuels virtually every cellular process, from muscle contraction to biochemical synthesis, making it an indispensable molecule. Yet, despite the clear importance of ATP, a major mystery remained—how was energy conserved rather than lost in the moment of release?
The search for answers spanned decades, as scientists investigated fermentation, respiration, and enzymatic activity to uncover the missing link in cellular energy conservation. The discovery of ATP synthesis transformed the field of bioenergetics, revealing a molecular reservoir that cells could tap into on demand. However, ATP production through respiration presented unexpected biochemical paradoxes. Researchers found that (1) electron transport and ATP synthesis appeared disconnected, (2) ATP yield fluctuated unpredictably, and (3) respiration was entirely dependent on an intact membrane. These contradictions defied conventional biochemical models, forcing a radical shift in thinking (see Box-1).
This article traces the scientific journey toward understanding ATP’s formation—debunking myths surrounding its so-called high-energy bond and unveiling the processes that sustain life at the molecular level. In doing so, it sets the stage for one of the most groundbreaking discoveries in bioenergetics: chemiosmotic coupling, a revolutionary concept pioneered by Peter Mitchell that would challenge long-held assumptions about cellular respiration and ATP production.
ATP: The Universal Energy Currency—A Story of Discovery
The quest to understand how cells conserve and utilize energy has fascinated scientists for centuries [1-5]. While David Keilin’s early concept of the respiratory chain correctly outlined electron transport, the fundamental question remained: how is energy stored rather than dissipated? The answer had to lie in an intermediate molecule capable of conserving and distributing this energy—one stable enough to exist until needed yet adaptable enough to fuel diverse cellular processes.
The first hints emerged from fermentation. Antoine Lavoisier, the father of modern chemistry, described fermentation as a simple chemical breakdown of sugar into alcohol and carbon dioxide, failing to recognize its deeper biological purpose. In the 19th century, fermentation became a battleground between vitalists, who believed in a special life force beyond chemistry, and reductionists, who insisted it was purely a molecular process. Louis Pasteur, a staunch vitalist, demonstrated that yeast cells actively ferment sugar in the absence of oxygen, famously calling fermentation “life without oxygen.” Despite proving its biological basis, Pasteur admitted he had no idea why yeast cells carried out fermentation.

Box-1. ATP: The Molecular Powerhouse of Life.
A breakthrough came in 1897 when Eduard Buchner shattered the belief that living cells were required for fermentation. By grinding German brewers’ yeast with sand and extracting a liquid that could ferment sugar, he proved that fermentation was driven by enzymes (from the Greek en zyme, meaning in yeast) rather than intact cells. His discovery of biological catalysts marked the end of vitalism and solidified the view that all living processes could be explained by biochemical principles.
Building on Buchner’s enzyme studies, Sir Arthur Harden and Hans von Euler later pieced together the sequence of reactions in fermentation. Their findings mirrored those of Otto Meyerhof, who, in 1924, showed that muscle cells follow nearly the same biochemical steps as yeast—though producing lactic acid instead of alcohol. Meyerhof’s work underscored the profound unity of life, revealing how fundamental metabolic pathways are shared across organisms, from yeast to humans.
By the late 1920s, scientists realized that cells used fermentation to generate energy when oxygen was scarce. The mystery deepened: was there a molecule acting as an energy currency, storing and distributing power throughout the cell? The answer emerged in 1929 when Karl Lohmann discovered ATP (adenosine triphosphate) in Heidelberg. ATP could be synthesized through fermentation and stored within cells, ready to release energy when needed. The molecule consisted of adenosine bound to three phosphate groups, with the terminal phosphate capable of breaking off and liberating energy.
Vladimir Engelhardt soon demonstrated ATP’s role in muscle contraction—without it, muscles remained rigid, as seen in rigor mortis. Fermentation, Engelhardt reasoned, must generate ATP to power biological work. However, fermentation produced only two ATP molecules per glucose, which raised another question: could respiration generate ATP more efficiently?
By the 1950s, Severo Ochoa confirmed that oxygen respiration far surpassed fermentation in ATP production, yielding up to 38 ATP molecules per glucose—a staggering 19-fold increase. Fritz Lipmann and Herman Kalckar further solidified ATP’s universal significance, declaring it the “energy currency of life.” Their bold claim held true, as ATP was found in every type of cell ever studied, from bacteria to plants to animals.
Ultimately, respiration, fermentation, and photosynthesis all converge on ATP, demonstrating a unifying principle of life. This elegant molecular system underscores how evolution optimized energy conservation, ensuring that every cell—whether microbial or human—is powered by the same universal currency.
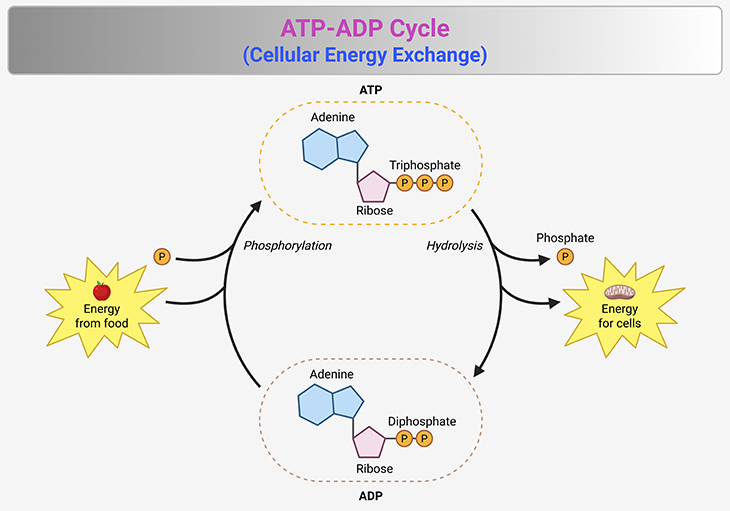
Figure 2. The ATP-ADP Cycle—Cellular Energy Exchange. This figure illustrates the ATP-ADP cycle, the fundamental energy exchange system that powers cellular processes. (1) ATP (adenosine triphosphate) serves as the primary energy carrier, undergoing continuous conversion to ADP (adenosine diphosphate) and inorganic phosphate (Pᵢ) through hydrolysis: ATP → ADP + Pᵢ + Energy (~30.5 kJ/mol or ~7.3 kcal/mol). This reaction releases energy that fuels essential biological functions, including muscle contraction, active transport, and biosynthesis. (2) The reverse process—ATP regeneration—requires an energy input, typically derived from cellular respiration, fermentation, or photosynthesis: ADP + Pᵢ + Energy (~30.5 kJ/mol or ~7.3 kcal/mol) → ATP. (3) ATP synthesis occurs primarily via ATP synthase, a rotary enzyme embedded in the mitochondrial inner membrane, which harnesses proton gradients to drive phosphorylation. The cycle maintains a high ATP-to-ADP ratio, ensuring a reservoir of readily available energy. This disequilibrium is actively sustained by metabolic pathways, preventing ATP from degrading to its natural equilibrium state. The ATP-ADP cycle exemplifies life’s dynamic energy management, enabling cells to efficiently store, transfer, and utilize energy in response to fluctuating demands.
The Myth of the Squiggle: Rethinking ATP’s High-Energy Bond
ATP has long been described as the cell’s energy reservoir, with its so-called “high-energy” bond often depicted using a squiggle (~) instead of a simple hyphen. The prevailing notion suggests that breaking this bond releases a large burst of energy, fueling cellular work. However, this interpretation oversimplifies reality—chemically speaking, ATP’s bonds are not exceptionally unique. The real secret lies in the equilibrium between ATP and ADP [6-7].
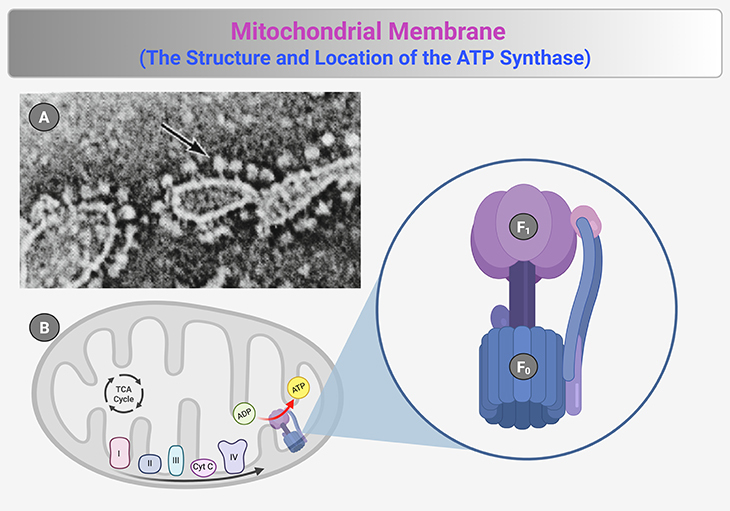
Figure 3. The Structure and Location of ATP Synthase. (1) ATP synthase, a molecular powerhouse driving ATP production, is strategically positioned on the mitochondrial inner membrane, where it converts energy stored in proton gradients into cellular fuel. Pioneering studies using electron microscopy revealed spherical particles protruding from membrane fragments, later identified as ATP synthase complexes. These particles, tethered by small stalk-like structures, were first observed in negatively stained mitochondrial membrane preparations [black arrow in panel (A)]—providing the earliest visual evidence of their existence. (2) Building on these findings, Efraim Racker and colleagues deciphered the structural and functional components of ATP synthase. Their work demonstrated that the spherical domain (F₁) exhibited ATP synthase activity, serving as the catalytic site for ATP production. Further investigations revealed that F₁ was anchored to a membrane-embedded complex (F₀), which facilitated proton translocation across the inner membrane [panel (B)]. This proton flow through F₀ powered the rotary mechanism of ATP synthase, driving the synthesis of ATP from ADP and inorganic phosphate (Pi). The discovery of ATP synthase’s intricate structure and localization provided a foundational understanding of cellular energy conversion, cementing its role as one of the most critical enzymatic complexes in bioenergetics. {Image credit (A) – modified and adapted from: Prebble JN. J Hist Biol. 2013 Winter;46(4):699-737.} [https://pubmed.ncbi.nlm.nih.gov/23104597/] [6].
In a test tube, ATP naturally breaks down into ADP and phosphate until equilibrium is reached. Yet, inside the living cell, the balance is dramatically reversed—nearly 90% of available ADP and phosphate is converted back into ATP (see Figure 2 and 3). This energy storage mechanism resembles a hydroelectric system: just as water is pumped uphill to store potential energy, ATP is maintained at high levels, awaiting the moment when cellular demand triggers its release to power vital processes.
ATP Production: The Enzyme Factories Behind Cellular Power
Understanding ATP’s formation led to pioneering discoveries in fermentation. In the 1940s, Efraim Racker, a towering figure in bioenergetics, uncovered how sugar breakdown generates phosphate-rich intermediates, which ultimately drive ATP formation. He envisioned a similar chemical coupling mechanism occurring in respiration, setting the stage for a long and frustrating pursuit of the elusive “high-energy intermediate.”
Racker later identified the ATP synthase (a.k.a. ATPase), a massive enzyme complex embedded in the mitochondrial membrane, responsible for ATP generation [8, 9]. Under the electron microscope, these structures appeared like tiny mushroom-like particles dotting the inner membrane (see Figure 3 and 4) [6]. Despite their proximity to respiratory chain complexes, they were not physically connected, raising a perplexing question: How did electron transport transfer energy to ATP synthase?
For decades, scientists searched for an intermediary molecule that would bridge this gap, one akin to the sugar-phosphate intermediate seen in fermentation. The problem was chemistry itself—biochemical reactions require physical contact, yet respiration and ATP synthesis appeared disconnected. The search for a “squiggle molecule” consumed an entire generation of researchers, producing and discarding over twenty potential candidates.
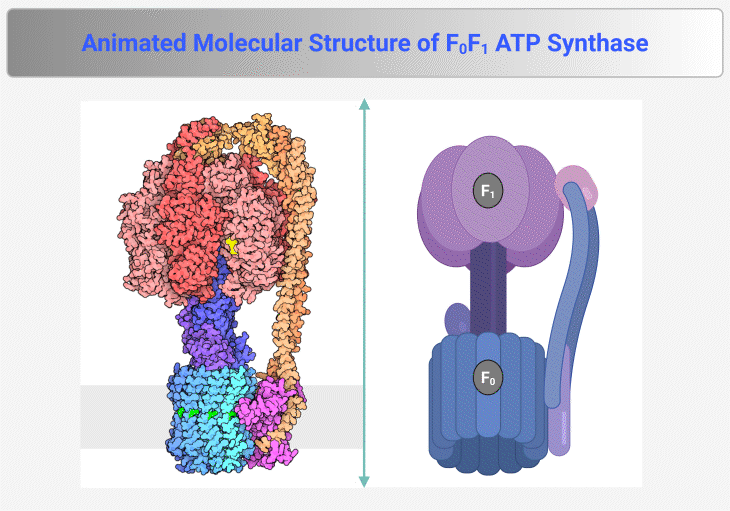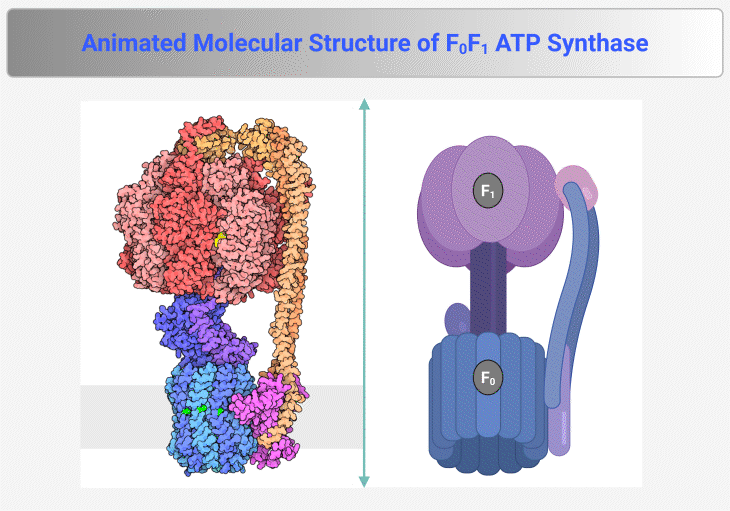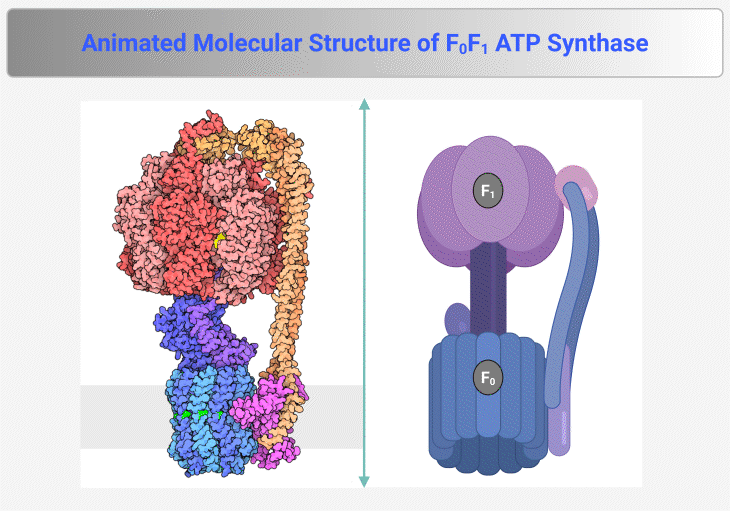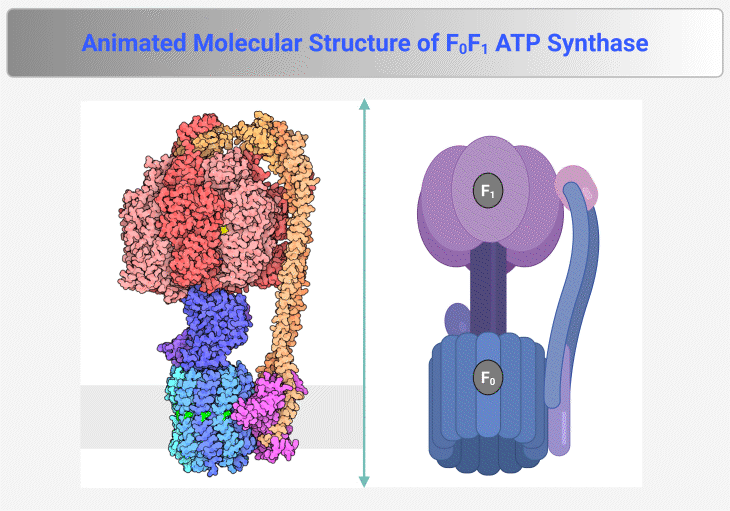
Figure 4. Molecular Structure of F₀F₁ ATP Synthase. This figure and animation illustrate the F₀F₁ ATP synthase, the rotary enzyme responsible for ATP production in mitochondria, chloroplasts, and bacteria. It consists of two main components: (1) F₀ Complex – Embedded within the membrane, this proton-translocating domain forms a channel that allows proton flow, generating rotational energy. (2) F₁ Complex – Extending into the mitochondrial matrix or cytoplasm, this catalytic domain harnesses rotational energy to convert ADP and inorganic phosphate (Pᵢ) into ATP. Proton translocation through F₀ induces conformational changes in F₁, driving ATP synthesis via chemiosmotic coupling. The enzyme operates at exceptional speed, synthesizing ATP at a rate of ~100 revolutions per second, ensuring a continuous energy supply for cellular processes. {Image credit – Animation from Exploring the Structural Biology of Bioenergy.} [PDB-101: PDB101.rcsb.org; PDB-101: Learn: Videos: ATP synthase]
The Biochemical Paradox and the Search for Order
A troubling inconsistency plagued ATP production in respiration—cells generated anywhere between 28 and 38 ATP molecules per glucose, a variation that defied basic chemical logic. Conventional chemistry relies on integer-based reactant ratios, yet ATP production operated on fluid, non-integer values. Another strange observation was that respiration required an intact membrane; if disrupted, ATP synthesis halted entirely, even when electron transport continued unhindered.
Certain molecules, known as uncouplers—including aspirin and even ecstasy—could disrupt ATP synthesis without physically damaging the membrane. The energy from electron transport dissipated as heat rather than being used to generate ATP, likened to a bicycle losing its chain—no matter how vigorously one pedals, the wheels refuse to turn. This phenomenon hinted that respiration and ATP synthesis were linked in a way beyond direct molecular interactions [8, 9].
By the early 1960s, researchers found themselves in a quagmire, unable to reconcile ATP synthesis with conventional biochemical rules. As Racker famously remarked, “Anyone who is not thoroughly confused just does not understand the problem.” The field was desperate for a breakthrough, and the radical answer came from Peter Mitchell in 1961. He proposed that cellular respiration operated through chemiosmotic coupling—a revolutionary concept that would redefine bioenergetics.
This paradigm shift will be explored in detail in the upcoming article: Proton Power.
Take-Home Messages
- ATP is life’s energy currency. No matter the organism—bacteria, fungi, plants, or animals—ATP powers essential cellular processes, unifying life’s biochemical foundation.
- Forget the “high-energy” bond myth. ATP’s strength is not in its chemical bonds but in its unnatural equilibrium—cells maintain a high ATP-to-ADP ratio, storing energy like a charged battery.
- Fermentation offered early clues but led scientists astray. Its phosphate intermediates helped uncover ATP formation but misled researchers into assuming respiration worked the same way.
- The hunt for the “high-energy intermediate” in respiration was futile. For decades, scientists chased an elusive molecule linking electron transport to ATP synthesis. It never existed.
- ATP synthase is a molecular powerhouse. This enzyme factory churns out ATP at staggering rates—about 70 kg daily in an average human—fueling everything from muscle contraction to metabolism.
- Membranes are essential for respiration. Break the membrane, and ATP synthesis halts, even as electron transport continues—a biochemical paradox that defied logic.
- Uncoupling breaks the system. Chemicals like aspirin and even ecstasy disrupt ATP production, causing energy to dissipate as heat instead—a phenomenon scientists struggled to explain.
- Peter Mitchell rewrote bioenergetics. His 1961 chemiosmotic coupling hypothesis shattered conventional thinking, revealing how respiration and ATP synthesis are linked by proton gradients—not direct chemical interactions.
This is where biology meets revolutionary thinking. Up next: Proton Power—the concept that changed everything.
(Cf. previous blog entitled as: “Cellular Respiration: The Hidden Engine Driving Life’s Energy.”)
Summary and Conclusions
ATP is the universal energy currency of life, fueling everything from metabolism to muscle contraction. It is synthesized through respiration, fermentation, and photosynthesis, demonstrating life’s profound biochemical unity. While ATP is often described as possessing a high-energy bond, its true power lies in the controlled disequilibrium inside cells—where ATP is maintained at concentrations far higher than its natural equilibrium, ensuring an ever-ready energy reservoir.
The journey to understanding ATP’s formation spanned decades of discovery and misdirection. Early studies on fermentation led scientists to believe that ATP synthesis followed a model of direct chemical coupling, where phosphate intermediates transferred energy in a straightforward reaction. This assumption misguided researchers in respiration, leading them on a prolonged search for a high-energy intermediate linking electron transport to ATP production—an intermediate that, in reality, never existed.
The breakthrough came with the discovery of ATP synthase (ATPase), a massive enzyme complex embedded within the mitochondrial membrane, responsible for ATP generation. However, a critical paradox emerged: ATPase and the respiratory chain were separated by a physical gap. How, then, did electron transport drive ATP synthesis? Conventional biochemical models dictated direct molecular interactions, yet none seemed to explain the process. Further compounding the mystery, ATP yield varied unpredictably, and respiration required an intact membrane—if disrupted, ATP synthesis ceased entirely, even as electron transport continued.
A final piece of the puzzle lay in uncoupling, a phenomenon where certain chemicals—including aspirin and even ecstasy—prevented ATP formation without physically damaging the mitochondrial membrane. Instead of producing ATP, respiration dissipated energy as heat, breaking the normal link between oxygen consumption and cellular energy production. These inconsistencies defied the principles of classical chemistry, forcing a complete re-evaluation of bioenergetics.
The radical answer arrived in 1961 when Peter Mitchell proposed chemiosmotic coupling, a groundbreaking hypothesis that reframed respiration. Rather than relying on direct molecular interactions, Mitchell demonstrated that proton gradients—created by electron transport—were the driving force behind ATP synthesis. This idea fundamentally changed our understanding of cellular energy, ranking alongside the discovery of the DNA double helix in its scientific significance.
Today, ATP remains one of biology’s most elegant molecules, unifying all forms of energy conversion. Whether through respiration, fermentation, or photosynthesis, life relies on ATP as its fundamental energy currency—a molecule whose discovery reshaped modern bioenergetics and continues to be central to understanding how cells sustain life.
For information on autism monitoring, screening and testing please read our blog.
References
- Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981 Dec;91(3 Pt 2):227s-255s. doi: 10.1083/jcb.91.3.227s. PMID: 7033239; PMCID: PMC2112799.
https://pubmed.ncbi.nlm.nih.gov/7033239/
https://discoveryandinnovation.com/papers/Schatz_1981.pdf
- Langen P, Hucho F. Karl Lohmann and the discovery of ATP. Angew Chem Int Ed Engl. 2008;47(10):1824-7. doi: 10.1002/anie.200702929. PMID: 18213664.
https://pubmed.ncbi.nlm.nih.gov/18213664/
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.200702929 - Engelhardt WA. Life and Science. Annu Rev Biochem. 1982;51:1-19. doi: 10.1146/annurev.bi.51.070182.000245. PMID: 6214208.
https://pubmed.ncbi.nlm.nih.gov/6214208/ - Emmerich M. Cells Flexing Their Muscles. MaxPlanckResearch 86-87 1|13. Max-Planck-Gesellschaft zur Förderung der Wissenschaften
https://www.mpg.de/7023399/S005_Flashback_086-087.pdf - Gest H. Landmark discoveries in the trail from chemistry to cellular biochemistry, with particular reference to mileposts in research on bioenergetics. Biochemistry and Molecular Biology Education 2002;30:9-13
https://iubmb.onlinelibrary.wiley.com/doi/full/10.1002/bmb.2002.494030010004 - Prebble JN. Contrasting approaches to a biological problem: paul boyer, peter mitchell and the mechanism of the ATP synthase, 1961-1985. J Hist Biol. 2013 Winter;46(4):699-737. doi: 10.1007/s10739-012-9343-7. PMID: 23104597.
https://pubmed.ncbi.nlm.nih.gov/23104597/
- Dreyfus BW, Sawtelle V, Turpen C, Gouvea J, Redish EF. Students’ reasoning about “high-energy bonds” and ATP: A vision of interdisciplinary education. Phys. Rev. ST Phys. Educ. Res. 10, 010115 – Published 12 May, 2014. doi: https://doi.org/10.1103/PhysRevSTPER.10.010115.
https://journals.aps.org/prper/pdf/10.1103/PhysRevSTPER.10.010115
- Yoshida M, Muneyuki E, Hisabori T. ATP synthase–a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol. 2001 Sep;2(9):669-77. doi: 10.1038/35089509. PMID: 11533724.
https://pubmed.ncbi.nlm.nih.gov/11533724/
- Noji H, Yoshida M. The rotary machine in the cell, ATP synthase. J Biol Chem. 2001 Jan 19;276(3):1665-8. doi: 10.1074/jbc.R000021200. Epub 2000 Nov 15. PMID: 11080505.
https://pubmed.ncbi.nlm.nih.gov/11080505/