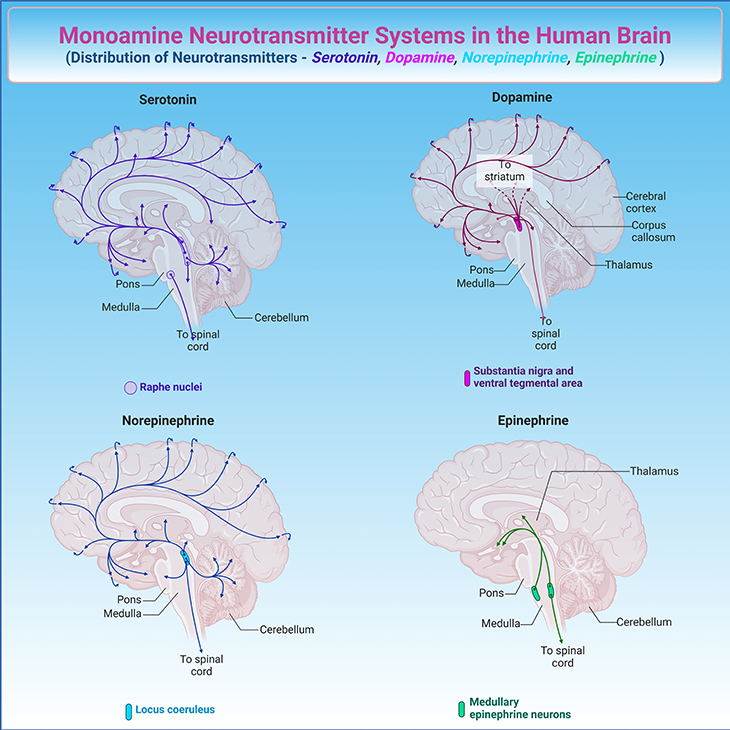
Figure 1. The Complex Web of Monoamine Neurotransmitter Pathways: Serotonin, Dopamine, Norepinephrine, and Epinephrine. The human brain is a marvel of intricate networks and pathways, with monoamine neurotransmitters playing a crucial role in regulating mood, cognition, and behavior. Let us explore the major projection pathways of serotonin, dopamine, norepinephrine, and epinephrine, and how they relate to the monoamine hypothesis of depression. 1. Serotonin (5-HT): Serotonergic neurons are primarily located in the raphe nuclei of the brainstem. These neurons project widely throughout the brain, including the cerebral cortex, hippocampus, amygdala, and spinal cord. Serotonin is involved in regulating mood, sleep, appetite, and pain. Disruptions in serotonergic pathways have been linked to mood disorders, including depression. 2. Dopamine (DA): Dopaminergic neurons are found in several key areas, including the substantia nigra and the ventral tegmental area (VTA). The major pathways include: (a) Nigrostriatal pathway: Projects from the substantia nigra to the striatum, involved in motor control. (b) Mesolimbic pathway: Projects from the VTA to the nucleus accumbens, involved in reward and motivation. (c) Mesocortical pathway: Projects from the VTA to the prefrontal cortex, involved in cognition and executive function. 3. Norepinephrine (NE): Noradrenergic neurons are primarily located in the locus coeruleus of the brainstem. These neurons project to various regions, including the cerebral cortex, hippocampus, thalamus, and spinal cord. Norepinephrine plays a role in arousal, attention, and stress response. 4. Epinephrine (Epi): Epinephrine-producing neurons are less abundant and are primarily located in the medulla oblongata. These neurons project to the hypothalamus, thalamus, and spinal cord. Epinephrine is involved in the fight-or-flight response and regulation of blood pressure. The “monoamine hypothesis of depression” posits that a deficiency in monoamine neurotransmitters—serotonin, dopamine, and norepinephrine—underlies the pathophysiology of depression. By examining the intricate pathways of monoamine neurotransmitters and their role in depression, we gain a deeper appreciation of the brain’s complexity and the challenges in treating mood disorders.
Introduction
Depression does not affect everyone equally. Some people are more susceptible than others, especially those facing tough life situations or those who have recently lost someone dear. But even within these groups, not everyone ends up clinically depressed. Scientists have developed various theories to explain these differences, including biological, psychological, and social factors. Here, we’ll take a closer look at some of these theories and how they come together to form a comprehensive understanding of depression.
The Serendipitous Discovery ~ Monoamine Hypothesis
Back in the mid-20th century, an unexpected discovery led to the development of the chemical imbalance model of depression. Doctors noticed that certain medications, initially used for other medical conditions, had surprising effects on patients’ moods. This observation paved the way for the monoamine hypothesis of depression, which we will explore in detail (see Figure 1) [1].
Brain Communication and Emotions: Our brains are incredibly complex, with various regions involved in controlling emotions. These regions communicate through the nervous system, which consists of neurons (nerve cells). Each neuron has a cell body, an axon (tail), and dendrites (branches). Neurons communicate by sending electrical impulses that trigger the release of chemical messengers called neurotransmitters across small gaps called synapses (see Figure 2).
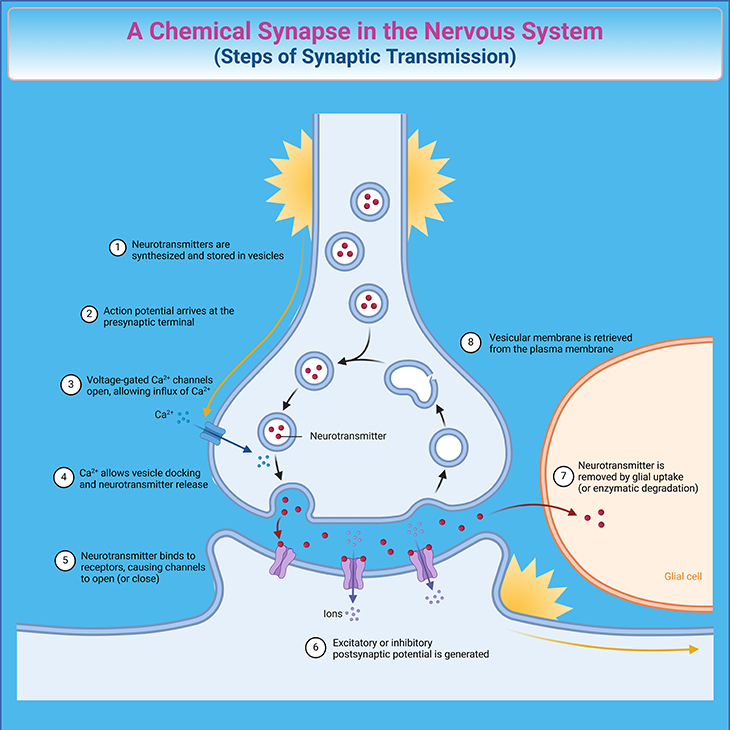
Figure 2. The Journey of Neural Messages: Understanding Synaptic Transmission. The process begins with the release of neurotransmitters into the synaptic cleft. The synapse includes various specialized areas where the presynaptic and postsynaptic neuronal membranes are closely aligned. Neurotransmitters are synthesized and stored in vesicles within the presynaptic neuron. When an action potential reaches the presynaptic axon terminals, it triggers the opening of voltage-gated Ca2+ channels, allowing Ca2+ to enter the cell. This influx of Ca2+ causes the vesicles to fuse with the presynaptic membrane, leading to the release of neurotransmitters into the synaptic cleft through exocytosis. The neurotransmitters then diffuse across the cleft and bind to receptor molecules on the postsynaptic neuronal membrane. This binding generates either excitatory or inhibitory postsynaptic potentials, depending on the type of neurotransmitter. Finally, the neurotransmitters are removed from the synaptic cleft by glial uptake or enzymatic degradation, and the vesicular membrane is retrieved and/or recycled from the plasma membrane.
Key Players – Monoamines: While there are many neurotransmitters, three monoamines – norepinephrine, dopamine, and serotonin – play a crucial role in depression. Serotonin regulates functions like sleep, eating, and mood. Norepinephrine is involved in stress reactions, alertness, and energy levels, as well as our interest in life. Dopamine influences motivation, pleasure, and reward-seeking behavior (see Figure 1).
A Tale of Two Drugs: In the 1950s, two medications, used for entirely different conditions, revealed fascinating insights into mood regulation. Reserpine, used to treat high blood pressure, caused significant depression in about 15 per cent of patients, sometimes accompanied by suicidal thoughts. On the other hand, iproniazid, used to treat tuberculosis, made patients feel happier, more energetic, and improved their appetite. Despite their different purposes, these drugs acted on the same neurotransmitter systems. Reserpine lowered monoamine levels, while iproniazid increased them by preventing the action of monoamine oxidase, an enzyme that breaks down monoamine neurotransmitters.
The Rise and Fall of the Monoamine Theory: The monoamine theory of depression gained popularity in the 1960s and 1970s. It suggested that a shortage of monoamines in the brain could explain depression symptoms. Researchers found differences in monoamine levels between depressed and non-depressed individuals in post-mortem studies. Experiments with drugs altering monoamine levels also supported this idea. Furthermore, studies of individuals who died by suicide revealed reduced monoamines in brain regions associated with emotional regulation. These findings led to the development of antidepressant medications that increased monoamine availability in synapses [2].
Critiques and Refinements: Despite its popularity, the monoamine hypothesis had its critics. They pointed out the danger of focusing on just a few neurotransmitters while ignoring the many others that also play roles in brain function. Animal studies showed that monoamines influenced multiple behaviors, not just those related to depression. Researchers acknowledged that the model was an oversimplification of a complex biological state. Even Paul Schildkraut, an early proponent of the theory, admitted that it was “undoubtedly, at best, a reductionist oversimplification.“
Challenges with Antidepressants: The monoamine theory led to the development of antidepressant medications, but their widespread use revealed flaws in the model. Not all medications altering monoamine levels produced the expected mood or behavioral effects. Moreover, there was often a two-week delay between increased monoamine levels and observable improvements in depressive symptoms, suggesting that these changes might be secondary effects of another primary biological process.
A New Perspective: Later revisions of the monoamine hypothesis shifted focus from the amount of neurotransmitter available in the synapse to receptor sensitivity, suggesting that deficiencies in the docking system might be more relevant. Scientists also began to explore the connections between neurotransmitter systems, other neural pathways, and the neuro-endocrine (hormonal) system.
Hormonal Harmony ~ The Neuro-Endocrine Powerhouse of Depression
Understanding how our bodies function can shed light on why some people are more susceptible to depression. One of the key biological models that explain this is the neuro-endocrine hypothesis, which looks at how hormones play a role in depression. People with endocrine disorders, like hypothyroidism, are often at a higher risk of experiencing depression.
The Hormonal Symphony: The endocrine system is a collection of organs such as the thyroid and adrenal glands that regulate many bodily functions by releasing hormones into the bloodstream. These hormones are produced in response to signals from the brain and their levels change predictably at different life stages (like puberty) and throughout the day (like the sleep-wake cycle). Unlike neurotransmitters, the first messengers in the chain between the brain and endocrine glands are called releasing factors, which are produced in the hypothalamus—a crucial brain region for hormone regulation. These releasing factors signal the pituitary gland to prompt hormone release from endocrine glands. Circulating hormones in the bloodstream influence various bodily processes and regulate the neuro-endocrine system, preventing hormone overproduction through a feedback loop (see Figure 3).
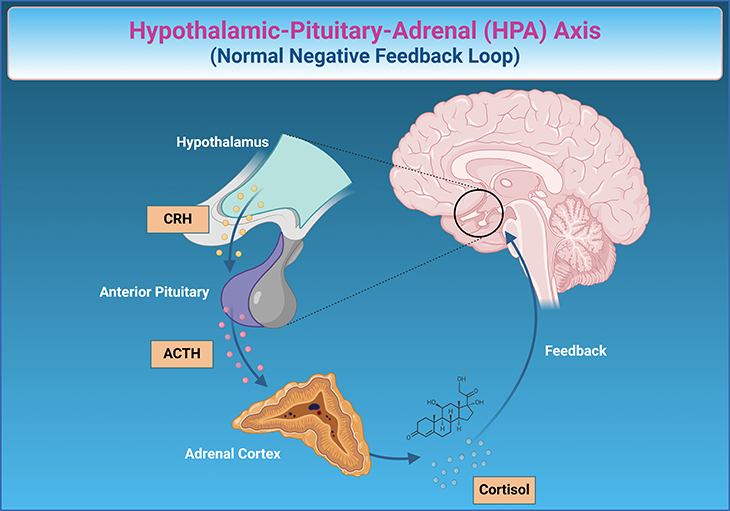
Figure 3. The Body’s Stress Symphony: Understanding the Hypothalamic-Pituitary-Adrenal (HPA) Axis in Depression. The hypothalamic-pituitary-adrenal (HPA) axis is a crucial system in your body that helps regulate your response to stress. Imagine it as a finely tuned orchestra involving three main sections: the hypothalamus, the pituitary gland, and the adrenal glands. When dealing with depression, the HPA axis often plays a significant role as part of the neuro-hormonal model of depression. Here is how it works in this context: When someone is experiencing depression, their brain perceives it as a form of chronic stress. The hypothalamus, which is responsible for initiating the stress response, sends a signal to the pituitary gland. This gland, often referred to as the “master gland,” releases a hormone called ACTH (adrenocorticotropic hormone) into the bloodstream. ACTH then travels to the adrenal glands, located above the kidneys, signaling them to produce and release cortisol, the body’s main stress hormone. In individuals with depression, this process can become dysregulated. The body might produce excess cortisol in response to prolonged stress, resulting in heightened stress levels that can exacerbate depressive symptoms. This chronic elevation of cortisol can lead to feelings of fatigue, anxiety, and difficulty concentrating—common features of depression. Ordinarily, the body uses a “negative feedback loop” to regulate this process. As cortisol levels rise, they signal back to the hypothalamus and pituitary gland to reduce the production of stress signals, essentially quieting the stress response. However, in depression, this feedback loop may be impaired. The hypothalamus and pituitary gland may become less sensitive to cortisol’s signals, leading to a continuous cycle of high cortisol levels and sustained stress response. Understanding this system highlights the intricate connection between our body’s stress response and mental health. By studying the HPA axis, researchers and clinicians hope to develop better treatments for depression, targeting these hormonal imbalances to help restore balance and improve overall well-being.
Brain and Hormone Connection: Receptors for serotonin, norepinephrine, and dopamine—all vital neurotransmitters—are present in the hypothalamus, indicating a connection between the monoamine system and hormone regulation. These neurotransmitter pathways link the amygdala and hippocampus, brain structures critical for emotional regulation, to the neuro-endocrine system. Hormone levels change in response to acute stress. For instance, adrenaline levels spike when a person faces anxiety-provoking situations, from public speaking to life-threatening events. This triggers the “fight or flight” response, causing increased heart rate, dizziness, nausea, and heightened vigilance [3].
Chronic Stress Response: The brain reacts differently to long-term stress or chronic adversity by producing a sequence of hormones. First, corticotrophin-releasing factor (CRF) is released from the hypothalamus. This, in turn, increases the production of adrenocorticotropic hormone (ACTH) from the pituitary gland, which regulates the release of cortisol, a key stress hormone, from the adrenal glands. Cortisol has extensive effects on the body, including metabolism and behavior, through its interaction with various brain regions (see Figure 3).
Normal versus Abnormal Stress Adaptation: Under normal circumstances, the body’s feedback loop ensures that hormone levels are regulated. However, in abnormal stress adaptation, this loop malfunctions. CRF levels become crucial in ‘fear conditioning’ and developing emotional memories of reward and punishment. Importantly, the HPA (hypothalamic-pituitary-adrenal) axis fails to turn off despite high cortisol levels, leading to a loss of the natural daily variation in cortisol levels. Persistently high cortisol levels can damage brain cells, speed up neuron death, and negatively impact memory and learning. Additionally, these high cortisol levels can reduce neurotransmitter levels linked to mood regulation [4].
Link to Clinical Depression: The changes in mood, appetite, and energy seen in abnormal stress responses mirror the core features of clinical depression. This has led researchers to propose that disrupted HPA axis functioning might be the underlying cause of depression. In the 1980s, there was hope that a test measuring HPA axis function and the feedback system (the dexamethasone suppression test) could diagnose depression. However, while both animal and human models of depression show HPA axis abnormalities, not all depressed individuals exhibit these abnormalities, and some people with HPA axis abnormalities have other mental health issues like anxiety, bipolar disorder, or post-traumatic stress disorder.
Ongoing Research: Despite the complexity of understanding how altered HPA axis function affects depression, it remains a significant focus for researchers worldwide. Many ongoing studies are exploring whether targeting this axis’s function might lead to new medications that can reduce depression risk or effectively treat its symptoms.
Take-Home Message
Individual Susceptibility
- Depression is more prevalent in individuals facing adverse social conditions or bereavement, but not everyone in these situations develops clinical depression.
Monoamine Hypothesis
- Initially discovered by chance, this hypothesis suggests that deficiencies in neurotransmitters like serotonin, norepinephrine, and dopamine could explain depression symptoms.
Chemical Messengers
- Neurotransmitters play a crucial role in brain communication. Serotonin regulates mood, sleep, and appetite; norepinephrine affects stress response and alertness; dopamine influences motivation and pleasure.
Medication Insights
- Observations of mood changes with certain medications led to the monoamine hypothesis and the development of antidepressants.
Limitations of Monoamine Theory
- Critiques highlight the oversimplification of focusing on a few neurotransmitters, while ignoring many others and not all medications altering monoamines produce expected effects.
Neuro-Endocrine Hypothesis
- Focuses on the role of hormones and the hypothalamic-pituitary-adrenal (HPA) axis in regulating stress responses and their link to depression.
Hormone Regulation
- Hormones produced in response to brain signals regulate various body functions and influence mood and behavior through the HPA axis.
Chronic Stress Impact
- Chronic stress disrupts the HPA axis feedback loop, leading to high cortisol levels, which negatively affect brain cells, memory, and mood.
Potential Diagnostic Test
- The dexamethasone suppression test aimed to diagnose depression by measuring HPA axis function, but not all depressed individuals show HPA abnormalities.
Ongoing Research
- Research continues to explore the biological underpinnings of depression, focusing on neurotransmitter and hormone regulation to develop better treatments.
(Cf. previous blogs entitled as: “Decoding Depression: Comprehensive Insights and Future Horizons.” “The Neurotypical Brain versus the Autistic Brain: Neurotypical Brain Chemistry”)
Summary and Conclusion
The article explores two pivotal biological models of depression: the Monoamine Hypothesis and the Neuro-Endocrine Hypothesis. The Monoamine Hypothesis suggests that a deficiency in specific neurotransmitters—namely norepinephrine, dopamine, and serotonin—could lead to depression. Initially discovered through observations of how certain medications affected mood, this hypothesis laid the groundwork for antidepressant development. Although popular in the mid-20th century, it faced criticism for its oversimplification and limited focus on a few neurotransmitters.
The Neuro-Endocrine Hypothesis emphasizes the role of hormones in depression, particularly through the hypothalamic-pituitary-adrenal (HPA) axis. This system links the nervous and endocrine systems, regulating stress responses. Chronic stress can disrupt the HPA axis’s feedback loop, leading to persistently high cortisol levels, which negatively impact brain cells, memory, and mood. Despite challenges in pinpointing exact causal mechanisms, the HPA axis remains a crucial area of ongoing research, with the potential to inspire new treatments for depression.
In conclusion, understanding the complex interplay of neurotransmitters and hormones is essential for unraveling the biological underpinnings of depression. While the Monoamine and Neuro-Endocrine Hypotheses have provided significant insights, further research is necessary to develop more effective interventions and improve mental health outcomes.
For information on autism monitoring, screening and testing please read our blog.
References
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61 Suppl 6:4-6. PMID: 10775017.
https://www.psychiatrist.com/jcp/history-evolution-monoamine-hypothesis-depression/ - Boku S, Nakagawa S, Toda H, Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018 Jan;72(1):3-12. doi: 10.1111/pcn.12604. Epub 2017 Oct 19. PMID: 28926161.
https://pubmed.ncbi.nlm.nih.gov/28926161/
- Li Z, Ruan M, Chen J, Fang Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci Bull. 2021 Jun;37(6):863-880. doi: 10.1007/s12264-021-00638-3. Epub 2021 Feb 13. Erratum in: Neurosci Bull. 2021 Jun;37(6):904. doi: 10.1007/s12264-021-00694-9. PMID: 33582959; PMCID: PMC8192601.
https://pubmed.ncbi.nlm.nih.gov/33582959/
- Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021 Sep 30;11(10):1298. doi: 10.3390/brainsci11101298. PMID: 34679364; PMCID: PMC8533829.
https://pubmed.ncbi.nlm.nih.gov/34679364/