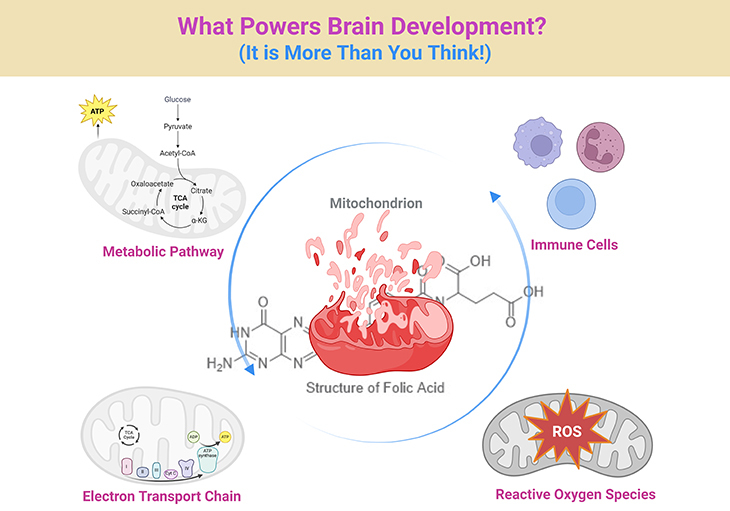
Figure 1. Decoding Development: Mitochondrial–Folate Signaling in Health and Autism Spectrum Disorder. We often hear that autism is “in the brain”—but how the brain builds itself is a story that begins deep within our cells. This simplified diagram traces how essential nutrients like folate and vitamin B12, together with our cells’ powerhouses—mitochondria—work together to shape brain growth, behavior, and health. When these cellular systems function well, they send the right signals to help the brain form connections, regulate immune responses, and handle daily stress. But when there is a glitch—due to genetics, environment, or nutrient imbalance—those signals can get scrambled, leading to challenges we recognize in autism spectrum disorder (ASD) and related health conditions. In this simplified visual guide, you will see how molecules become messages—and how scientists and clinicians are learning to restore balance by supporting the body’s own language of development. (1) Neurodevelopmental Homeostasis: In the healthy state, mitochondrial oxidative phosphorylation (Complexes I–V) supports efficient ATP generation, redox stability, and formate flux from the mitochondrial folate cycle (via SHMT2, MTHFD2). One-carbon units fuel nucleotide synthesis, SAM production for DNA/histone methylation, and glutathione (GSH) synthesis for antioxidant defense. Balanced outputs regulate neural progenitor proliferation, synaptic maturation, and immune quiescence. (2) Pathophysiology in ASD: ASD-associated disruptions include impaired electron transport chain (ETC) activity—particularly Complex I and IV—leading to reduced ATP, increased ROS, and release of mitochondrial DAMPs (e.g., mtDNA). Simultaneous folate transport deficits (e.g., FRα autoantibodies, MTHFR polymorphisms) result in decreased methylation potential and glutathione imbalance. These changes skew microglial activation, upregulate inflammatory pathways (TLR9, NLRP3, cGAS–STING), and alter gene expression in developing neurons. Clinical manifestations span cognitive, behavioral, gastrointestinal, and immune dysregulation. Thus, this integrative depiction highlights metabolism as an active messaging system—translating nutrient, redox, and genetic inputs into neurodevelopmental outcomes. [SHMT2, serine hydroxymethyltransferase 2; MTHFD2, a bifunctional enzyme – methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2, methenyltetrahydrofolate cyclohydrolase; Complex I-V, different enzyme complexes (I–V) of the electron transport chain (ETC); SAM, S-adenosine methionine; DNA, deoxyribonucleic acid; GSH, glutathione; ASD, autism spectrum disorders; ATP, adenosine triphosphate; ROS, reactive oxygen species; DAMPs, damage-associated molecular patterns; mtDNA, mitochondrial DNA; FRα, folate receptor-alpha; MTHFR, methylenetetrahydrofolate reductase; TLR9, Toll-like receptor 9; NLRP3, NLR family pyrin domain containing 3; cGAS-STING, cyclic GMP-AMP synthase-stimulator of interferon genes].
Metabolism as Message: Rethinking Autism Through the Lens of Cellular Signaling
For decades, autism spectrum disorder (ASD) has been categorized and dissected through neurobehavioral frameworks, often with the goal of identifying behavioral phenotypes or uncovering unifying genetic markers. But despite major advances in genomics and brain imaging, a consistent theme has emerged: ASD is profoundly heterogeneous, defying singular explanations. As this complexity continues to unfold, one concept is rapidly gaining traction across disciplines—that metabolism is not simply the backdrop to development, but a central signaling language through which neurobiological identity is formed and altered.
Among the emerging messengers in this cellular lexicon, mitochondrial function and folate-mediated one-carbon metabolism have stepped into the spotlight. Recent clinical and molecular studies suggest that these systems do far more than fuel neurons and clear reactive oxygen species. Instead, they act as integrative platforms that encode environmental input, direct epigenetic decisions, and modulate immune responses. When disrupted—as is increasingly documented in subsets of individuals with ASD—these pathways may skew developmental timing, synaptic architecture, and systemic physiology (see Figure 1; Box-1) [1, 2].
This is not theoretical. Data from cohorts of children with ASD indicate that up to 80% exhibit biomarkers of mitochondrial dysfunction, particularly within Complex I and IV of the electron transport chain (ETC) [3, 4]. Muscle biopsies, lymphocyte respiration assays, and cerebrospinal fluid (CSF) lactate levels routinely reveal deficits in oxidative phosphorylation. These findings are accompanied by elevated markers of oxidative stress and redox imbalance—conditions known to impair neurogenesis and alter synaptic function.
In parallel, disruptions in folate and B12 metabolism—especially via MTHFR polymorphisms, impaired folate receptor function, or cerebral folate deficiency (CFD)—are being recognized as both risk modifiers and clinical targets. Children with FRα autoantibodies, for instance, often present with regressive autism, seizures, and language delays. When treated with leucovorin (a.k.a. folinic acid or 5-formyl tetrahydrofolic acid), some demonstrate striking improvements in communication and social engagement, suggesting a powerful interplay between folate signaling and neural function [5].
But these systems do not act in isolation. The mitochondrial folate cycle, facilitated by enzymes such as SHMT2 and MTHFD2, integrates cellular proliferation cues with epigenetic needs. During early development, flux through this cycle helps determine whether one-carbon units are used for (1) nucleotide synthesis (supporting mitosis), (2) formate production (fueling methylation reactions), or (3) glutathione regeneration (buffering oxidative stress). These decisions shape not only DNA integrity but transcriptional landscapes—especially in rapidly developing tissues like the fetal brain.
This perspective reframes ASD not solely as a neurodevelopmental disorder, but as a metabolically guided systems condition, in which neural, immune, and gut-brain axes are modulated by shifts in mitochondrial and folate signaling. Consider the following loop:
- Mitochondrial dysfunction impairs ATP production and redox regulation
- This triggers neuroinflammation and epigenetic dysregulation
- Which in turn amplifies oxidative stress, weakening mitochondrial resilience further
- Leading to systemic symptoms: seizures, sleep disturbance, GI dysmotility, developmental regression
Importantly, this loop is not a death spiral—it is a therapeutic entry point. Clinical interventions that address (a) mitochondrial support (e.g., CoQ10, carnitine, riboflavin), (b) folate transport (e.g., folinic acid), and (c) redox rebalancing (e.g., vitamin C, N-acetylcysteine [NAC]) are already showing impact [6]. These are not silver bullets—but in carefully selected individuals, they represent precision interventions grounded in cellular reality (see Figure 1; Box-1).
What this calls for is a paradigm shift. We must begin to view metabolism not as static chemistry, but as an active system of interpretation. Like neurons responding to neurotransmitters, cells interpret metabolic inputs as information: what to become, how to respond, whether to divide or die. When that information stream is distorted—through genetic mutations, environmental stressors, or disrupted nutrient pathways—the outcome may be behavioral, cognitive, and systemic changes we recognize as part of the autism spectrum [7].
This view does not negate the value of behavioral therapies, educational interventions, or genetic insight. It simply completes the picture, offering clinicians and researchers a deeper framework through which to interpret heterogeneity, comorbidity, and response variability.
To truly support those on the autism spectrum, we must expand our diagnostic lens from phenotype to pathway—from surface to signal. The mitochondrion, the folate cycle, and the redox matrix are not peripheral—they are the first interpreters of developmental experience. By listening more closely to their messages, we may just change the future of neurodevelopmental care.
Clinical Case Vignette: Metabolic Insight in Pediatric Autism

Box-1. Clinical Case Vignette: Metabolic Insight in Pediatric Autism.
(Cf. previous blogs entitled as: “Defining Autism Spectrum Disorders: Mechanisms of Developmental Regression in Autism.”; “Defining Autism Spectrum Disorders: Mechanisms of Developmental Regression in Autism – II.”; “Cellular Respiration: The Hidden Engine Driving Life’s Energy.”; “The Brain on Food: Rethinking Mental Health from the Inside Out.”)
Take-Home Message
- Autism spectrum disorder (ASD), once viewed primarily through behavioral lenses, is now increasingly understood as a condition rooted in complex metabolic and mitochondrial interplay. At the heart of this landscape lies the folate–methionine–vitamin B12 axis—a biochemical pathway responsible for DNA synthesis, methylation, and redox regulation. These processes shape the trajectory of early neurodevelopment, and their disruption leaves deep systemic imprints.
- Emerging studies reveal that many individuals with ASD exhibit disruptions in the one-carbon metabolism pathway, manifesting as elevated homocysteine, low folate or B12, and altered SAM/SAH ratios. Such imbalances are not mere laboratory anomalies—they disturb the very machinery of neuronal maturation, synaptic pruning, and epigenetic regulation. Polymorphisms in genes like MTHFR or autoantibodies against folate receptor alpha (FRα) obstruct cerebral folate transport, compounding the neurological burden.
- Downstream, mitochondrial dysfunction frequently coexists with these deficits. Impaired oxidative phosphorylation, excess reactive oxygen species (ROS), and mtDNA instability are not just cellular curiosities—they are mechanistic hallmarks found in the brains, muscles, and gastrointestinal tracts of individuals with ASD. What emerges is not a single broken node, but a systemic failure to maintain energy homeostasis in developing tissues.
- These disruptions do not act in isolation. Seizures, gastrointestinal disturbances, and neurodevelopmental regression often co-occur—tethered by impaired mitochondrial resilience and the inability to meet local energy demands during growth spurts or inflammatory states.
- Crucially, this landscape is not without therapeutic promise. Folinic acid (leucovorin), methyl-B12, and antioxidant support have shown positive impact in children with demonstrable metabolic vulnerabilities—particularly those with cerebral folate deficiency (CFD) or mitochondrial markers. These interventions suggest a future where metabolic profiling may guide personalized therapies, moving beyond diagnostic labels to functional repair.
- In sum, the convergence of folate/B12 metabolism and mitochondrial dynamics offers not just insight into the pathogenesis of ASD, but a hopeful pathway toward intervention—one illuminated by biochemistry, shaped by clinical nuance, and sustained by the resilience of developing minds.
For information on autism monitoring, screening and testing please read our blog.
References
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012 Mar;17(3):290-314. doi: 10.1038/mp.2010.136. Epub 2011 Jan 25. PMID: 21263444; PMCID: PMC3285768.
https://pubmed.ncbi.nlm.nih.gov/21263444/ - Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, Tassone F, Pessah IN. Mitochondrial dysfunction in autism. JAMA. 2010 Dec 1;304(21):2389-96. doi: 10.1001/jama.2010.1706. PMID: 21119085; PMCID: PMC3915058.
https://pubmed.ncbi.nlm.nih.gov/21119085/ - Napoli E, Wong S, Hertz-Picciotto I, Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014 May;133(5):e1405-10. doi: 10.1542/peds.2013-1545. PMID: 24753527; PMCID: PMC4006429.
https://pubmed.ncbi.nlm.nih.gov/24753527/ - Frye RE, Slattery JC, Quadros EV. Folate metabolism abnormalities in autism: potential biomarkers. Biomark Med. 2017 Aug;11(8):687-699. doi: 10.2217/bmm-2017-0109. Epub 2017 Aug 3. PMID: 28770615.
https://pubmed.ncbi.nlm.nih.gov/28770615/ - Zhang C, Chen Y, Hou F, Li Y, Wang W, Guo L, Zhang C, Li L, Lu C. Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients. 2025 May 7;17(9):1602. doi: 10.3390/nu17091602. PMID: 40362912; PMCID: PMC12073535.
https://pubmed.ncbi.nlm.nih.gov/40362912/ - Delhey LM, Tippett M, Rose S, Bennuri SC, Slattery JC, Melnyk S, James SJ, Frye RE. Comparison of Treatment for Metabolic Disorders Associated with Autism:Reanalysis of Three Clinical Trials. Front Neurosci. 2018 Feb 12;12:19. doi: 10.3389/fnins.2018.00019. PMID: 29483858; PMCID: PMC5816043.
https://pubmed.ncbi.nlm.nih.gov/29483858/ - Kovacheva E, Gevezova M, Mehterov N, Kazakova M, Sarafian V. The Intersection of Mitophagy and Autism Spectrum Disorder: A Systematic Review. Int J Mol Sci. 2025 Feb 28;26(5):2217. doi: 10.3390/ijms26052217. PMID: 40076836; PMCID: PMC11899999.
https://pubmed.ncbi.nlm.nih.gov/40076836/




