Table of Contents
- Introduction
- The Metabolic Architecture of Motherhood
- Metabolic Marvels: How Pregnancy Reshapes Maternal Physiology
- Lactation: The Metabolic Art of Nourishing New Life
- The Science of Lactation: Fueling Infancy with Precision
- Take-Home Messages
- Summary and Conclusions
- Did You Know About Folate Receptor Autoantibodies (FRAAs) and Brain Development?
- References
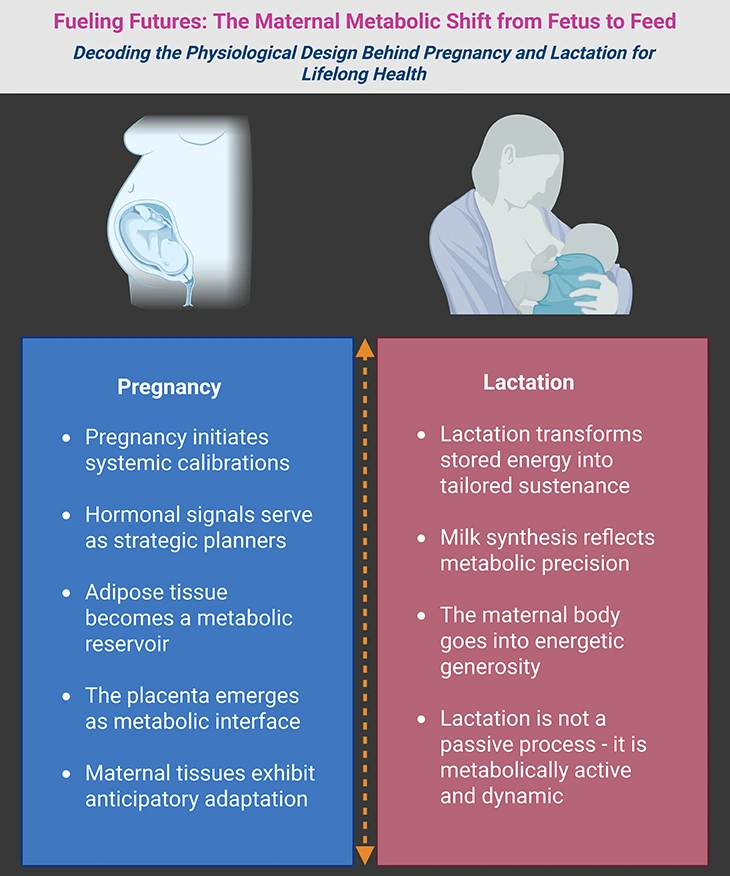
Figure 1. Fueling Futures: The Maternal Metabolic Shift from Fetus to Feed. Imagine the maternal body as a dynamic engine—quietly recalibrating, storing, and transforming energy to sustain new life. (1) On the left, we see the pregnancy phase, where metabolism shifts gears to support fetal growth. Hormones act as strategic planners, guiding nutrient storage and vascular expansion. Adipose tissue becomes a reservoir, and the placenta emerges as a metabolic interface—delivering oxygen, glucose, and amino acids with precision. (2) On the right, the lactation phase unfolds. The body now mobilizes its reserves, converting stored energy into milk—a substance rich in carbohydrates, fats, proteins, and immune factors. The mammary gland becomes a biochemical factory, responding to hormonal cues like prolactin and oxytocin. Milk synthesis is not passive—it is metabolically active, demanding glucose, lipids, and amino acids in finely tuned proportions. (3) For expectant mothers, this story affirms the wisdom of their bodies—how every craving, breath, and heartbeat contributes to a future unfolding within. For clinicians, it is a reminder of the intricate physiology that underpins maternal care—where each metabolic shift is an opportunity to support health across generations.
Introduction

Box-1a. Metabolic Lullaby: A Song of Maternal Adaptation.
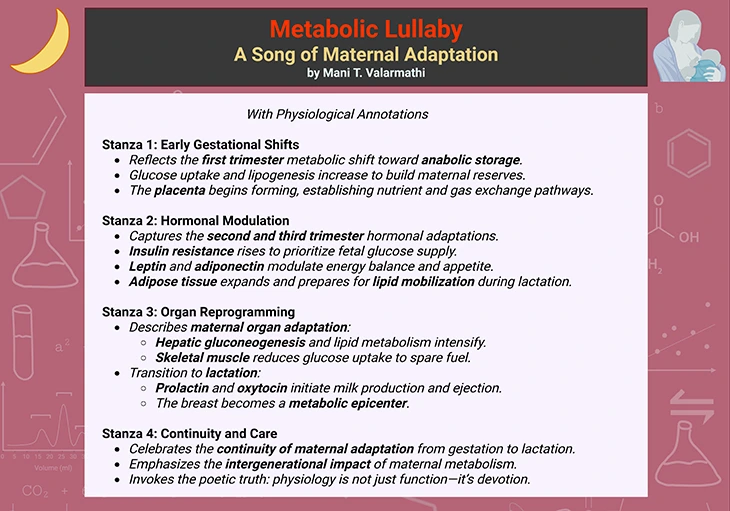
Box-1b. Metabolic Lullaby: A Song of Maternal Adaptation
(with physiological annotations).
The Metabolic Architecture of Motherhood
Pregnancy and lactation are not simply biological events—they are feats of physiological engineering. From the earliest stages of gestation to the sustained nourishment of a newborn, the maternal body undergoes a sweeping redesign of its metabolic landscape. Every system is recalibrated, every resource reallocated, to support the creation and care of new life.
This transformation is neither abrupt nor chaotic. It unfolds with remarkable precision, guided by hormonal signals, nutrient flows, and cellular decisions that reflect millions of years of evolutionary refinement. The maternal body becomes a living scaffold—supporting fetal development, storing energy, and later converting those reserves into milk tailored for the infant’s growth and immunity.
In this article, we explore the structural and functional shifts that define maternal metabolism during pregnancy and lactation. From the rising energy demands of gestation to the biochemical intricacies of milk production, we trace how the body adapts, prioritizes, and sustains (see Box-1a and 1b). Whether you are a clinician, scientist, or curious reader, this journey reveals the maternal body as a dynamic system—resilient, responsive, and exquisitely tuned to the needs of the next generation.
I. Metabolic Marvels: How Pregnancy Reshapes Maternal Physiology
Pregnancy is one of the most metabolically demanding phases in a mammal’s life. For humans, this period involves a remarkable reconfiguration of maternal energy use, nutrient allocation, and hormonal orchestration—all to support the growth of a new life (see Figure 1) [1, 2].
Energy Economics of Pregnancy
Over the course of a typical pregnancy, a woman consumes an additional 370 megajoules (MJ)—equivalent to 88,400 kilocalories (kcal). This substantial energy investment reflects a profound shift in maternal metabolism. By the third trimester, a pregnant woman’s total energy expenditure (TEE) rises to about 11.5 MJ per day, compared to 9.9 MJ per day in a non-pregnant woman of similar body composition. That is a 15% increase, or roughly 380 extra kcal daily (see Figure 2).
This energy demand places most pregnant women in a state of positive energy balance, often described as anabolic—where the body builds and stores tissue. However, this balance can fluctuate depending on the stage of pregnancy and nutritional status.
Breaking Down Energy Use
TEE during pregnancy includes:
- Basal Metabolic Rate (BMR) – the energy used at rest
- Diet-Induced Thermogenesis (DIT) – the energy used to digest and process food
- Activity Energy Expenditure (AEE) – the energy spent on physical movement
In pregnancy, there is an additional energy cost for synthesizing new tissue—both protein and fat. On average, women gain 12.5–13.5 kg, including:
- 700–900 g of newly synthesized maternal protein
- 3.8–4.3 kg of fat
This is energy retained in the body, not just spent.
BMR on the Rise
BMR increases progressively through pregnancy (human pregnancy is divided into three equal parts, or trimesters):
- 4% in the first trimester
- 10% in the second
- Up to 24% in the third trimester
This rise is driven by tissue growth and increased workload on the heart, lungs, and kidneys. Of the 370 MJ total energy cost, nearly 160 MJ (over 38,000 kcal) is attributed to elevated BMR alone (see Figure 2).
Curiously, some well-nourished women show a decrease in BMR during the first trimester—a phenomenon not yet fully understood. These changes appear to be influenced by pre-pregnancy body fat levels. Additionally, levels of free triiodothyronine (T3)—a key thyroid hormone—tend to decrease, possibly as a compensatory mechanism to regulate BMR.
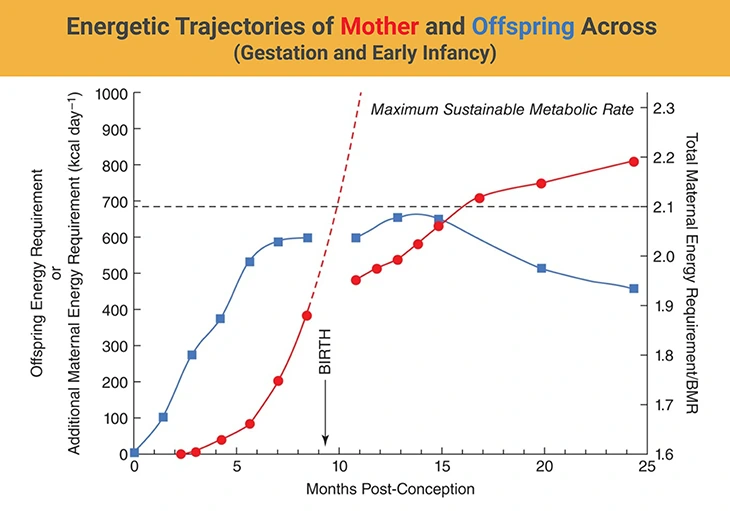
Figure 2. Energetic Trajectories of Mother and Offspring Across Gestation and Early Infancy. This figure illustrates the evolving energy demands of the fetus (red circles) and the mother (blue squares) throughout human pregnancy and into early postnatal life. Fetal energy requirements rise exponentially across gestation, peaking near term. In contrast, maternal energy expenditure increases during early pregnancy but plateaus by the end of the second trimester, stabilizing at approximately twice the basal metabolic rate (BMR)—as shown on the right-hand axis (Total Energy Requirement/BMR). The dashed line projects a hypothetical continuation of fetal energy demand beyond nine months, highlighting that such a trajectory would exceed sustainable maternal capacity. After birth, neonatal energy needs grow gradually, while maternal energy expenditure remains capped at around 2× BMR, reflecting the metabolic balance achieved during lactation. {Image credit – modified and adapted from: Dunsworth et al. Metabolic hypothesis for human altriciality. Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15212-6. https://pubmed.ncbi.nlm.nih.gov/22932870/}
Thermogenesis and Movement
Changes in DIT are generally minimal and remain proportionally stable relative to TEE. As for physical activity, energy expenditure does not change significantly, although some evidence suggests that metabolic efficiency during exercise may improve during pregnancy.
A Primate Advantage
Humans, like other primates, experience slow fetal development, which spreads the energy cost over a long 9-month gestation. This results in lower energy stress compared to other mammals. In well-nourished women, the maintenance cost of pregnancy (sustaining the fetus and associated tissues) is about four times higher than the cost of synthesizing those tissues.
Interestingly, while fetal weight accounts for only 25% of maternal weight gain, fat deposition contributes to 40% of TEE. The remaining one-third is used for metabolic maintenance. Across cultures and nutritional backgrounds, fat gain during pregnancy is relatively protected, even when food is scarce. In contrast, BMR can be down-regulated in response to poor nutrition—an adaptive strategy that helps safeguard fetal development.
Hormonal Symphony: Orchestrating Metabolic Shifts
Despite the low overall energy stress, metabolic changes are essential to meet fetal demands. These shifts begin early—within the first 10 weeks—and are likely driven by hormonal changes.
Most metabolic substrates in maternal blood decrease during pregnancy, due to both expanded plasma volume and increased utilization. One key exception is glucose, which remains elevated to fuel the fetus (see Box-2). Maternal tissues become insulin-resistant, likely due to hormones like:
- Human placental lactogen (hPL) – also known as human chorionic somatomammotrophin, structurally similar to growth hormone
- Estrogens – which also influence carbohydrate and lipid metabolism
This insulin resistance enhances glucose delivery to the placenta. Meanwhile, plasma triacylglycerol levels nearly double by term (see Box-2) [3-5].
Protein Priorities
Protein metabolism undergoes dramatic changes:
- Nitrogen conservation increases, especially in the third trimester, reducing urinary nitrogen loss
- Plasma amino acid concentrations drop by about 20%, reflecting increased placental uptake
- Maternal branched-chain amino acid oxidation decreases
These changes support both fetal growth and maternal tissue synthesis, although the precise hormonal mechanisms remain unclear.
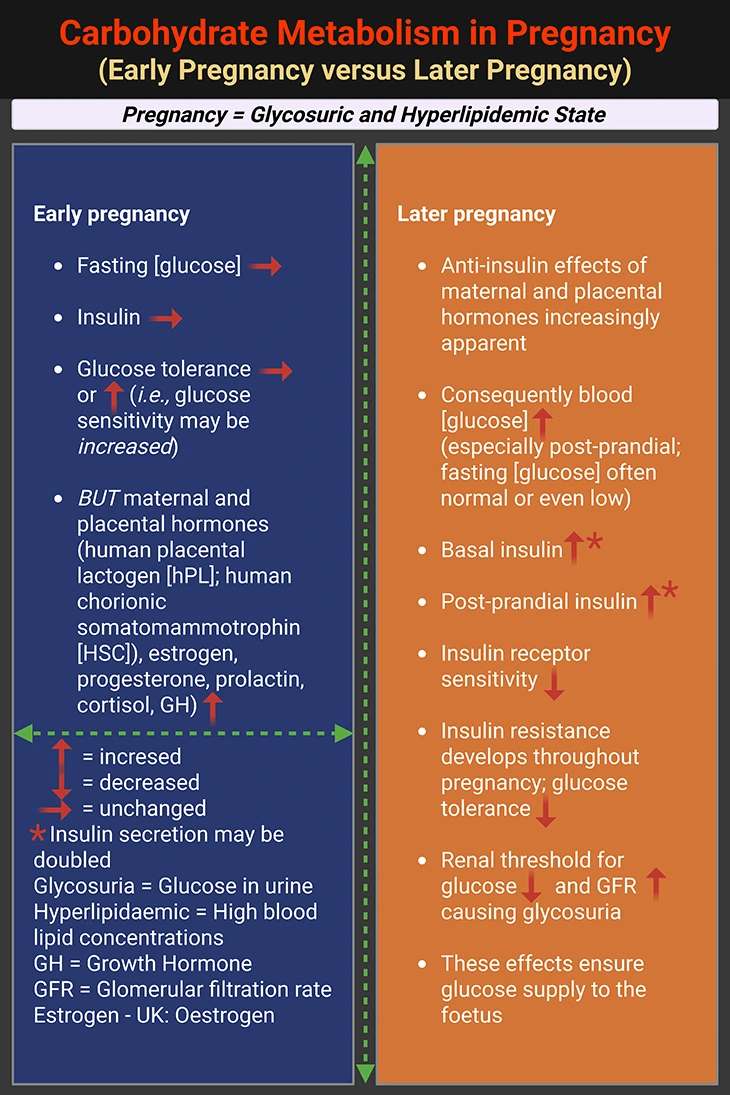
Box-2. Carbohydrate Metabolism in Pregnancy. {Box credit – modified and adapted from: Human Metabolism: A Regulatory Perspective, 4th Edition (2019). Keith N. Frayn and Rhys D. Evans. Companion website for resources: (www.wiley.com/go/frayn)}
II. Lactation: The Metabolic Art of Nourishing New Life
Among all the body’s specialized tissues, the mammary gland stands out for its extraordinary ability to export nutrients. While muscles are designed to burn fuel and fat stores it, the mammary gland transforms maternal resources into milk—a substance uniquely tailored to support infant development. This process, known as lactation, is a defining feature of mammals (from the Latin mamma, meaning breast, and noun form, maternal), and it represents one of the most metabolically demanding feats in biology.
Milk as a Metabolic Export
Human milk production averages around 750 milliliters per day, though it can exceed 1 liter daily during peak lactation. Each gram of milk contains approximately 2.8 kilojoules (kJ), or 0.67 kilocalories (kcal), translating to a daily energy cost of about 2.5 MJ—roughly 600 kcal. This is a substantial metabolic investment, especially considering that milk is the sole source of nutrition for infants.
While impressive in humans, this export pales in comparison to rodents. Mice and rats, for example, produce enough milk during their 21-day lactation cycle to grow their entire litter to the size of the mother herself. To achieve this, the lactating rodent must double its food intake, a feat of extreme metabolism. In humans, the 2.6 MJ/day energy cost of lactation (assuming 80% efficiency) is met primarily through diet—about 1.9 MJ/day (nearly 500 kcal)—with the remainder drawn from maternal energy stores.
Interestingly, well-nourished women tend to mobilize body reserves more readily than undernourished mothers. Yet, despite nutritional differences, milk production remains remarkably consistent across populations, unless conditions reach famine-level deprivation.
Metabolic Adjustments: Subtle, Not Sweeping
Are there other metabolic strategies to ease the burden of lactation? In humans, not significantly. While some studies suggest possible changes in basal metabolic rate (BMR), thermic effect of feeding, or exercise efficiency, the evidence is mixed and inconclusive. The body’s primary support system remains its energy stores, shaped by nutritional status at the end of pregnancy and dietary intake during lactation.
Typically, a well-nourished woman loses about 0.8 kg per month while breastfeeding, whereas an undernourished mother may lose only 0.1 kg monthly. Importantly, mobilizing body reserves is helpful but not essential for successful lactation.
Milk Composition: A Tailored Nutritional Package
Milk is a complete food, containing carbohydrates, fats, proteins, vitamins, and minerals. While minerals and vitamins will not be discussed here, the macronutrient profile of human milk is worth noting (see Table 1 and Table 2):
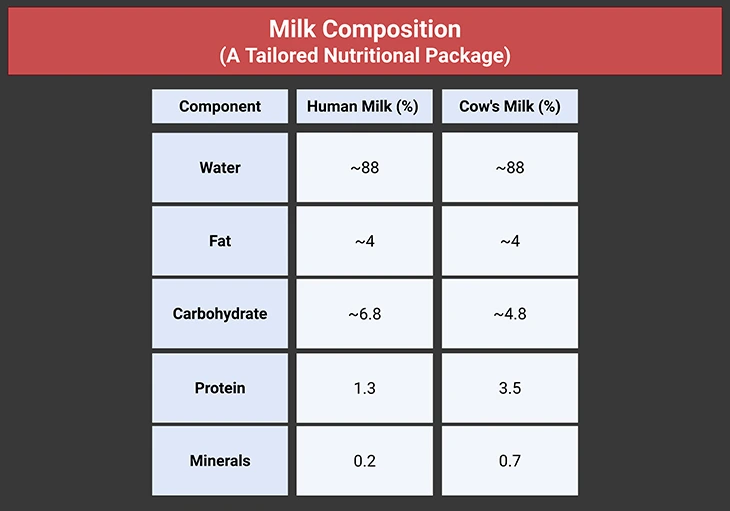
Table 1. Milk Composition: A Tailored Nutritional Package.
Human milk contains 50% more carbohydrate than cow’s milk, but less than half the protein and one-third the minerals. This difference has raised concerns when cow’s milk is used to replace breast milk in infants. However, milk composition varies widely across species. For instance, grey seals produce milk with over 50% fat, reflecting their unique ecological needs. The enormous variation in milk composition between different species is illustrated in Table 2.
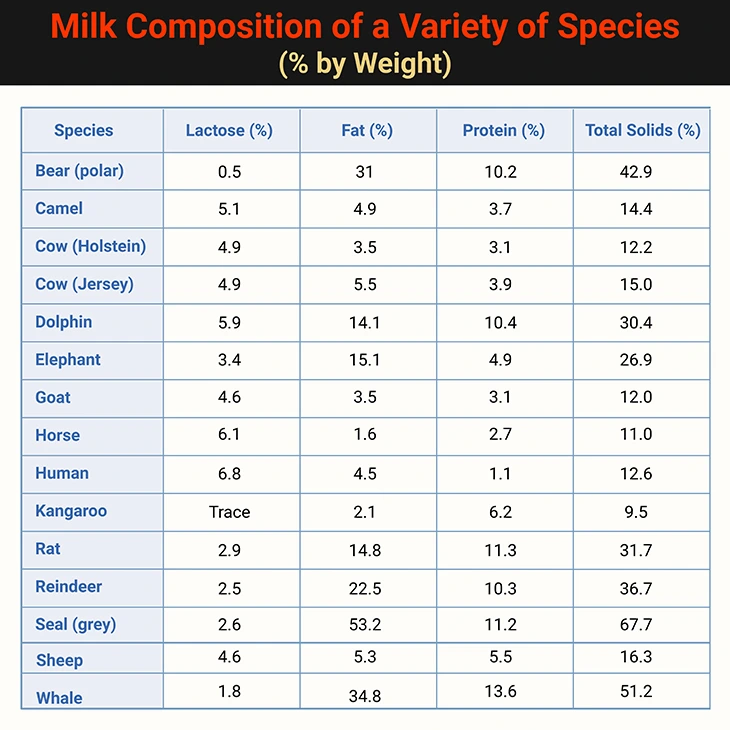
Table 2. Milk Composition of a Variety of Species (% by Weight). The difference between the combined substrates and the total solids is the “ash,” that is the mineral content. {Table credit – modified and adapted from: Human Metabolism: A Regulatory Perspective, 4th Edition (2019). Keith N. Frayn and Rhys D. Evans. Companion website for resources: (www.wiley.com/go/frayn)}
Hormonal Control: The Symphony Behind Milk Production
Lactation is tightly regulated by hormones, with a well-orchestrated sequence of events beginning in pregnancy [3]:
- Estrogen (from the placenta) stimulates ductal development in the breast.
- Progesterone promotes growth of lobules and alveoli.
- Growth hormone, glucocorticoids, and insulin support gland development.
However, estrogen and progesterone also inhibit milk secretion during pregnancy. This phase, known as Stage I lactogenesis or secretory differentiation, is further supported by prolactin and human placental lactogen (hPL).
After birth, the placenta is expelled, causing a sharp drop in estrogen and progesterone. This lifts the inhibition, triggering Stage II lactogenesis, now called secretory activation. Prolactin and hPL surge, driving milk production. Prolactin levels remain elevated during lactation, with spikes during suckling.
Other essential hormones include:
- Insulin
- Glucocorticoids
- Thyroxine (likely involved)
Let-Down Reflex: The Final Step
Milk production is only part of the story—milk ejection is equally vital. This reflex is triggered by nipple stimulation, which activates the hypothalamus and prompts the posterior pituitary to release oxytocin. Oxytocin causes myoepithelial cells around the alveoli to contract, pushing milk into the ducts for the infant to consume.
III. The Science of Lactation: Fueling Infancy with Precision
Lactation is not just a biological marvel—it is a finely tuned metabolic process that transforms maternal resources into a complete nutritional package for the newborn. From the first drops of colostrum to the sustained production of mature milk, the mammary gland orchestrates an extraordinary export of energy and nutrients [6-9].
Colostrum: Immunity in a Bottle
In the first few days after birth, the mammary gland produces colostrum—a specialized form of milk low in fat but rich in proteins, particularly immunoglobulin A (IgA). This antibody plays a crucial role in establishing the infant’s gut-associated immune system, offering protection during a vulnerable period of early life.
Lactose: The Sweet Backbone of Milk
By day two or three, milk composition shifts. Lactose, a disaccharide made of glucose and galactose, becomes the dominant carbohydrate, providing about 40% of the milk’s energy. Lactose is synthesized in the Golgi apparatus of mammary epithelial cells using up to 60 g of glucose per day, transported via the GLUT-1 transporter. The enzyme lactose synthase facilitates this process.
Because lactose is hydrophilic, it draws water into secretory vesicles, helping determine the volume and hydration of milk. In fact, glucose availability may be a key limiting factor in how much milk a mother can produce. About two-thirds of glucose taken up by the mammary gland is used for lactose synthesis, with additional glucose supporting lipogenesis and NADPH production via the pentose phosphate pathway.
Milk Fat: Energy and Essential Lipids
Milk fat, primarily triacylglycerol, contributes most of the energy in human milk—about 50 g per day. It also carries fat-soluble vitamins. Fat content varies widely across and within species, but human milk is relatively low in fat.
About half of the milk’s triacylglycerol comes from very-low-density lipoproteins (VLDL) and chylomicrons in maternal blood, imported into mammary cells via lipoprotein lipase (LPL)—which reaches its highest activity in the lactating mammary gland. The rest is synthesized de novo within the gland, likely regulated by SREBP-1c.
In species that rely heavily on dietary fat, the fatty acid profile of milk mirrors the maternal diet. In contrast, species that synthesize most of their milk fat internally show more consistent, species-specific profiles. In humans:
- Short-chain fatty acids (≤10 carbons) are entirely synthesized in the gland.
- Long-chain fatty acids (≥18 carbons) are imported from plasma lipids.
Human milk contains notable levels of polyunsaturated fatty acids (PUFAs) like docosahexaenoic acid (DHA, 22:6 n-3) and arachidonic acid (AA, 20:4 n-6). These are thought to support neonatal brain development, though the benefits of maternal fish oil supplementation remain under investigation.
Other components under active research for their role in cognitive development include:
- Choline
- Sialic acid – a key part of gangliosides, which are sphingolipids found in the brain
Most milk sterols, including cholesterol, are derived from maternal hepatic synthesis and incorporated into milk via lipoproteins. Since milk is mostly water, its lipids form micelles, similar to plasma lipoproteins. These micelles float to the top of standing milk, forming cream, while the remaining liquid becomes skimmed milk.
Milk Proteins: Builders and Defenders
Milk proteins fall into two major categories (see Table 3):
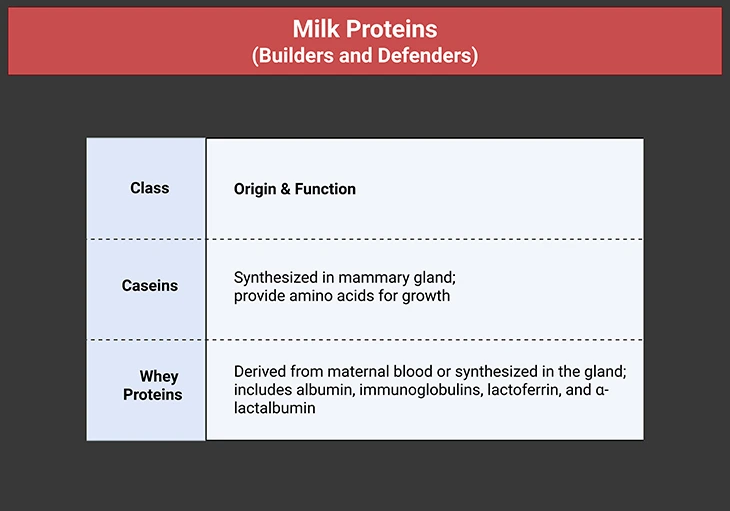
Table 3. Milk Proteins Builders and Defenders. They comprise two main classes – the caseins (from the Latin caseus – cheese) and the whey proteins (from the Old English hwœġ – to pile up, build).
Caseins are tailored for neonatal digestion and growth. Their synthesis involves upregulation of amino acid transporters in mammary epithelial cells. Whey proteins, including α-lactalbumin, play regulatory roles. α-Lactalbumin is the regulatory subunit of lactose synthase, preventing galactose polymerization and ensuring efficient lactose production (see Table 3).
Hormonal Regulation: A Delicate Balance
Lactation is governed by a complex hormonal network:
- Prolactin and oxytocin are central to milk production and ejection.
- Insulin, glucocorticoids, and possibly thyroxine support synthesis and secretion.
The mammary gland is highly insulin-sensitive, responding to feeding by ramping up production of lactose, fat, and protein. In contrast, low insulin levels during starvation quickly suppress lactation—a maternal survival mechanism.
Interestingly, prolactin induces insulin resistance in other maternal tissues, ensuring that nutrients are prioritized for milk production. This contrasts with pregnancy, where insulin sensitivity increases, promoting fat storage in preparation for lactation.
Glucocorticoids are essential for lactation, but elevated levels due to stress can inhibit milk production and ejection.
Autocrine Control: Milk Demand Drives Milk Supply
The continuation of lactation—called galactopoiesis—depends not just on hormones but also on milk removal. If milk is regularly expressed, production continues. If suckling stops, lactation quickly ceases (involution). This autocrine feedback loop complements endocrine regulation.
Experiments in goats showed that frequently milked udders maintained production, while unused udders stopped producing milk. This suggests the presence of an inhibitory factor—possibly 5-hydroxytryptamine (5-HT) or FIL (feedback inhibitor of lactation)—that builds up when milk is not removed, suppressing further synthesis. While the exact identity of this factor remains elusive, its role in regulating lactation is clear.
Take-Home Messages
Pregnancy: Metabolic Foundations in Motion
- Pregnancy initiates a systemic recalibration, where maternal metabolism shifts from self-maintenance to fetal provisioning.
- Hormonal signals serve as strategic planners, orchestrating nutrient storage, vascular remodeling, and insulin sensitivity to prepare for future demands.
- Adipose tissue becomes a metabolic reservoir, accumulating energy not just for gestation, but for the energetically demanding phase of lactation.
- The placenta emerges as a metabolic interface, regulating nutrient transfer, hormonal communication, and immune modulation between mother and fetus.
- Maternal tissues exhibit anticipatory adaptation, adjusting their metabolic priorities in preparation for the dual roles of nurturing and nourishing.
Lactation: The Metabolic Symphony of Nourishment
- Lactation transforms stored energy into tailored sustenance, with the mammary gland acting as a biochemical factory of growth and immunity.
- Milk synthesis reflects metabolic precision, integrating hormonal cues, nutrient availability, and cellular machinery to meet neonatal needs.
- The maternal body enters a phase of energetic generosity, mobilizing lipid stores and enhancing glucose utilization to sustain milk production.
- Lactation is not a passive process—it is metabolically active and dynamic, requiring continuous coordination across endocrine and metabolic pathways.
- Understanding lactation metabolism reveals a blueprint for resilience, showcasing how maternal physiology balances depletion with renewal.
Summary and Conclusions
Maternal metabolism during pregnancy and lactation represents one of the most profound physiological transformations in human biology. Across these phases, the body reconfigures its energy priorities, hormonal signaling, and tissue functions to support the developing fetus and later, the growing infant. Pregnancy initiates a preparatory phase marked by increased insulin resistance, enhanced lipogenesis, and strategic nutrient storage—particularly in adipose tissue. These changes are orchestrated by a complex hormonal milieu, including rising levels of placental lactogen, estrogen, progesterone, and insulin, which collectively recalibrate maternal physiology to favor fetal growth and placental function.
Lactation, in turn, activates a distinct metabolic program. The mammary gland becomes a central site of nutrient transformation, converting maternal stores into milk rich in lipids, lactose, and immunological components. This phase is characterized by heightened lipolysis, increased glucose uptake, and sustained hormonal regulation via prolactin and oxytocin. Importantly, lactation is not merely a continuation of pregnancy metabolism—it is a metabolically demanding phase that requires its own adaptive strategies, including shifts in mitochondrial function and nutrient partitioning.
Despite these well-characterized transitions, several gaps in knowledge remain. The precise molecular mechanisms that govern tissue-specific metabolic rewiring—particularly in adipose, hepatic, and mammary tissues—are still being elucidated. Emerging evidence suggests that mitochondrial dynamics, epigenetic modifications, and maternal microbiome shifts may play underappreciated roles in shaping metabolic outcomes. Moreover, inter-individual variability in metabolic adaptation, influenced by genetics, diet, and environmental exposures, remains a critical area for investigation.
Future research must also address the long-term implications of maternal metabolic states on offspring health. The concept of developmental programming—wherein maternal nutrition and metabolism influence lifelong risk of metabolic disorders in the child—demands deeper exploration. Integrating omics technologies, longitudinal cohort studies, and translational models will be essential to unravel these complex intergenerational links.
In conclusion, maternal metabolism is not a static process but a dynamic continuum of adaptation, resilience, and biological ingenuity. By deepening our understanding of these transitions, we not only illuminate the architecture of motherhood but also unlock new avenues for improving maternal and neonatal health across diverse populations.
For information on autism monitoring, screening and testing please read our blog.
References
-
- Zeng Z, Liu F, Li S. Metabolic Adaptations in Pregnancy: A Review. Ann Nutr Metab. 2017;70(1):59-65. doi: 10.1159/000459633. Epub 2017 Mar 16. PMID: 28297696.
https://pubmed.ncbi.nlm.nih.gov/28297696/ - Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000 Mar;54 Suppl 1:S47-51. doi: 10.1038/sj.ejcn.1600984. PMID: 10805038.
https://pubmed.ncbi.nlm.nih.gov/10805038/ - Rassie KL, Giri R, Melder A, Joham A, Mousa A, Teede HJ. Lactogenic hormones in relation to maternal metabolic health in pregnancy and postpartum: protocol for a systematic review. BMJ Open. 2022 Feb 21;12(2):e055257. doi: 10.1136/bmjopen-2021-055257. PMID: 35190436; PMCID: PMC8860010.
https://pubmed.ncbi.nlm.nih.gov/35190436/ - Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002 Oct;19(1):43-55. doi: 10.1385/ENDO:19:1:43. PMID: 12583601.
https://pubmed.ncbi.nlm.nih.gov/12583601/ - Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med. 2009 Apr;14(2):66-71. doi: 10.1016/j.siny.2008.09.004. Epub 2008 Nov 4. Erratum in: Semin Fetal Neonatal Med. 2009 Dec;14(6):401. PMID: 18986856.
https://pubmed.ncbi.nlm.nih.gov/18986856/ - Özlem Şengül1, Suat Dede. Maternal and Fetal Carbohydrate, Lipid and Protein Metabolisms. Eur J Gen Med 2014; 11(4):299-304. DOI : 10.15197/sabad.1.11.93
https://www.ejgm.co.uk/download/maternal-and-fetal-carbohydrate-lipid-and-protein-metabolisms-7180.pdf - Anhê GF, Bordin S. The adaptation of maternal energy metabolism to lactation and its underlying mechanisms. Mol Cell Endocrinol. 2022 Aug 1;553:111697. doi: 10.1016/j.mce.2022.111697. Epub 2022 Jun 8. PMID: 35690287.
https://pubmed.ncbi.nlm.nih.gov/35690287/ - Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005 Oct;8(7A):1010-27. doi: 10.1079/phn2005793. PMID: 16277817.
https://pubmed.ncbi.nlm.nih.gov/16277817/ - Mennitti LV, Oliveira JL, Morais CA, Estadella D, Oyama LM, Oller do Nascimento CM, Pisani LP. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem. 2015 Feb;26(2):99-111. doi: 10.1016/j.jnutbio.2014.10.001. Epub 2014 Oct 12. PMID: 25459884.
https://pubmed.ncbi.nlm.nih.gov/25459884/
- Zeng Z, Liu F, Li S. Metabolic Adaptations in Pregnancy: A Review. Ann Nutr Metab. 2017;70(1):59-65. doi: 10.1159/000459633. Epub 2017 Mar 16. PMID: 28297696.