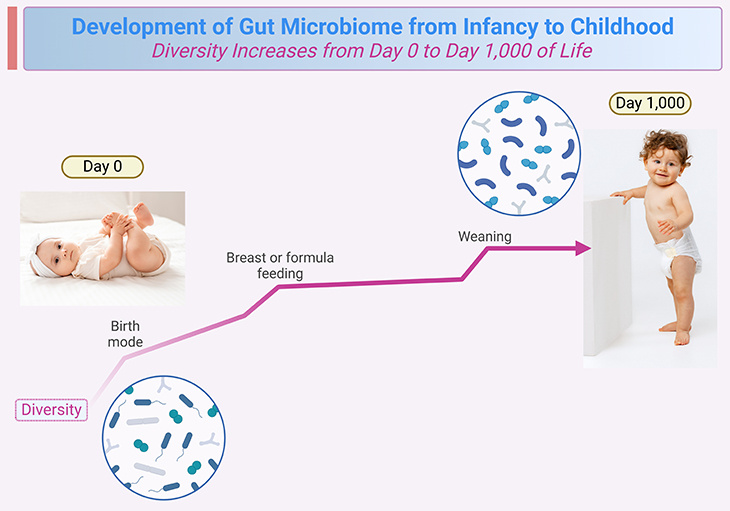
Figure 1. The schematic shows that diversity increases from day 0 to day 1,000 of life. The development of the gut microbiome from infancy to childhood is indeed a fascinating and complex process. Birth mode, initial feeds, and weaning are major events in which the diversity increases more rapidly.
Introduction
Neonatal and, to a lesser degree, infant gut microbiome development is chaotic, dynamic, and highly individual. As discussed in our previous blogs, the temporal development of the bacterial community during this time can be influenced significantly by environmental, host-related, and clinical variables. By 1 to 3 years of age there is generally less clinical intervention, and the gut microbiome reaches a “climax community” of microbes that will generally remain stable through adulthood (see Figure 1) [1-3]. (Cf. previous blogs entitled as: ‘Developmental Origins of Health and Disease: Neonatal Gut Microbiome ~ Day 0 to 30.’; and ‘Developmental Origins of Health and Disease: Infant Gut Microbiome ~ Day 31 to 364.’)
Although there is no specific gut microbiome in adults, compared to neonates and infants there is a notable increase in the Bacteroidetes at the expense of Proteobacteria, while Firmicutes remains relatively dominant from birth. The more adultlike gut microbiome is predominantly characterized by increased abundances of the following genera:
- Clostridium,
- Faecalibacterium,
- Blautia,
- Ruminococcus,
- Lactobacillus (Firmicutes),
- Bacteroides, and
- Prevotella (Bacteroidetes), as well as
- Bifidobacterium.
The exact age at which the adult-like community is established is not yet known and likely varies between individuals, especially driven by nutritional status and geography, with Western populations reaching maturation by 1 year of life.
In a study comparing the gut microbiome of children aged 0 to 3 years between three extreme groups – (i) American Indians from the Amazon, (ii) Rural Malawians, and (iii) urban American – the bacterial profiles were distinct between the groups, but the Western American children were the greatest outliers [4]. Notably, Bifidobacteria still dominated the communities from all locations.
Although the gut microbiome is still developing, there are many factors contributing to the microbes that colonize it. Aside from the geographical and dietary extremes earlier, more subtle influences within a given population may have profound long-term effects. Having siblings results in a higher proportion of Bifidobacterium compared to single infants [5].
Perhaps unsurprisingly, the gut microbiomes of twins are more comparable to each other than those of other nonrelated infants, but it is unclear if this is driven by comparable environmental exposure of genetic influence. Exposure to animals, such as household pets, will also shape the developing gut microbiome.
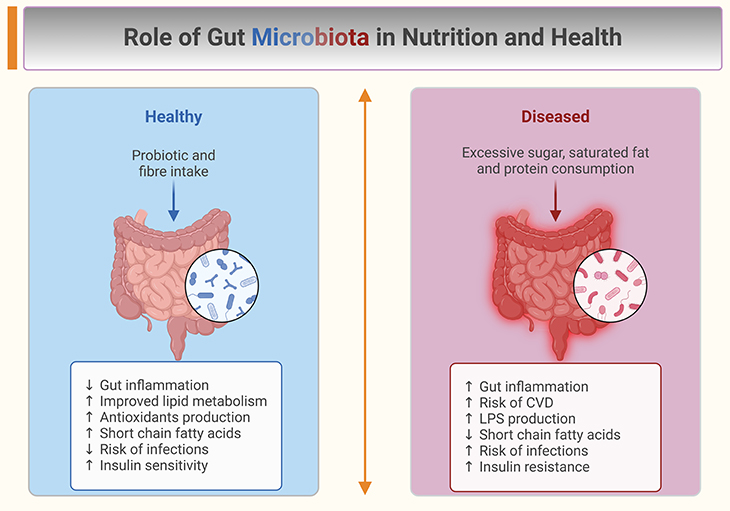
Figure 2. Role of gut microbiota in nutrition and health. Schematic representation of the role of the gut microbiota in health and disease giving some examples of inputs and outputs: (i) Gut microbiota influences many areas of human health from innate immunity to appetite and energy metabolism; (ii) Targeting the gut microbiome with probiotics or dietary fiber, benefits human health and could potentially reduce obesity; (iii) Drugs, food ingredients, antibiotics, and pesticides could all have adverse effects on the gut microbiota; (iv) Microbiota should be considered a key aspect in nutrition – the medical community should adapt their education and public health messages; (v) Fiber consumption is associated with beneficial effects in several contexts. [CVD, cardiovascular disease; LPS, lipopolysaccharide] (Adapted and modified from: Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018 Jun 13;361:k2179. Doi: https://doi.org/10.1136/bmj.k2179) [6].
A recent study showed that the gut microbiome profiles of preterm infants at 1 to 3 years of age was comparable to that of term infants in terms of both diversity and the abundant bacterial genera [7]. The same study also found no profound lasting effects on the gut microbiome (a) from a previous diagnosis of disease, (b) number of days of antibiotics, (c) gestational age, (d) birth mode, and (e) mode of delivery.
These findings are further supported by a recent study, which found that the same variables do not influence the gut microbiome long term, with the additional findings that maternal weight also has no lasting effect.
There is currently a progressive increase in metabolic and immune-mediated diseases in infants and children for which the developing gut microbiome might have important consequences (see Figure 2). Studies exploring the gut microbiome in allergic disease and obesity have risen in recent years, but the direct effect of the gut microbiome on these conditions is not well determined. Still, it is reasonable to postulate that the gut microbiome will have important influences.
For example, a reduced abundance of Bacteroides, which is common to the Western diet associated with low dietary fiber intake, was shown to predict atopic eczema (see Figure 2). Likewise for obesity, a reduced abundance of Bifidobacterium and an increased prevalence of Staphylococcus was found at 1 year of age in infants who went on to become obese at 7 years of age.
The neonatal and infant phases seem the most applicable for modulation of the gut microbiome. However, in certain circumstances, such as following antibiotic administration, there may be clear rationale for seeding a beneficial gut microbiome through the use of probiotics and prebiotics.
Take Home Messages
Introduction
- Neonatal and infant gut microbiome: Highly dynamic and individual, influenced by environmental, host-related, and clinical factors.
- Stabilization: By ages 1 to 3, the gut microbiome generally stabilizes into a “climax community” of microbes that remains relatively stable through adulthood.
Adult-like gut microbiome
- Composition and key genera: By 1 to 3 years of age, the gut microbiome generally stabilizes into an adult-like community, characterized by specific bacterial genera such as Clostridium, Faecalibacterium, Bacteroides, and Bifidobacterium.
Influencing factors
- Geography and diet: Western populations reach microbiome maturation by 1 year, with distinct bacterial profiles compared to other populations.
- Siblings and pets: Having siblings or pets can influence the composition of the gut microbiome.
- Preterm infants: By ages 1 to 3, their gut microbiome is comparable to that of term infants, with no lasting effects from early clinical variables.
Health implications
- Metabolic and immune diseases: The developing gut microbiome may influence the risk of diseases like allergies and obesity.
- Predictive factors: Reduced Bacteroides is linked to atopic eczema, while reduced Bifidobacterium and increased Staphylococcus are linked to obesity.
Summary and Conclusions
Although microbiome research has offered unprecedented information on the role of microbes in health and disease, the field as a whole is still emerging. Further work is needed to ascertain the extent to which microbiome findings are causative and the subsequent mechanism, proving causality over existing correlations. The next decades offers huge promise for microbiome research, no more so than for the developing infant, where the first 1,000 days offers a key window to establish a beneficial population contributable to long-term health.
For information on autism monitoring, screening and testing please read our blog.
References
- Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The Human Microbiome and Child Growth – First 1000 Days and Beyond. Trends Microbiol. 2019 Feb;27(2):131-147. doi: 10.1016/j.tim.2018.09.008. Epub 2018 Oct 24. PMID: 30529020.
https://pubmed.ncbi.nlm.nih.gov/30529020/ - Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017 Nov 8;81(4):e00036-17. doi: 10.1128/MMBR.00036-17. PMID: 29118049; PMCID: PMC5706746.
https://pubmed.ncbi.nlm.nih.gov/29118049/ - Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017 Oct;66(4):515-522. doi: 10.1016/j.alit.2017.07.010. Epub 2017 Aug 18. PMID: 28826938.
https://pubmed.ncbi.nlm.nih.gov/28826938/ - Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012 May 9;486(7402):222-7. doi: 10.1038/nature11053. PMID: 22699611; PMCID: PMC3376388.
https://pubmed.ncbi.nlm.nih.gov/22699611/
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006 Aug;118(2):511-21. doi: 10.1542/peds.2005-2824. PMID: 16882802.
https://pubmed.ncbi.nlm.nih.gov/16882802/ - Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018 Jun 13;361:k2179. doi: 10.1136/bmj.k2179. PMID: 29899036; PMCID: PMC6000740.
https://pubmed.ncbi.nlm.nih.gov/29899036/ - Stewart CJ, Skeath T, Nelson A, Fernstad SJ, Marrs EC, Perry JD, Cummings SP, Berrington JE, Embleton ND. Preterm gut microbiota and metabolome following discharge from intensive care. Sci Rep. 2015 Nov 24;5:17141. doi: 10.1038/srep17141. PMID: 26598071; PMCID: PMC4657104.
https://pubmed.ncbi.nlm.nih.gov/26598071/