Table of Contents
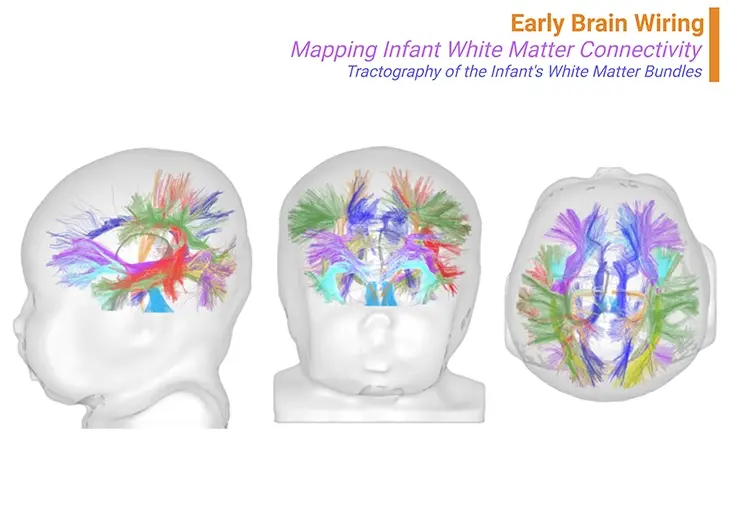
Figure 1. Early brain wiring ~ Mapping infant white matter connectivity. Beneath the cortex, a crucial event in brain development occurs – its wiring. At birth, the brain’s major bundles of connections are already in place, their growth primarily guided by genetics. However, the final terminations of these connections are not fully established. Axons must seek out their targets over years, a process heavily influenced by experience. Learning shapes these neural pathways, synapse by synapse. Using tractography based on fiber orientation distribution functions (fODE) and other methods, scientists can reconstruct the trajectories of main white matter bundles—projections, callosal tracts, limbic, and associative bundles—providing ‘virtual dissections,’ as shown in the brain of a 1-month-old infant. {Adapted and modified from: Dubois et al., 2020 J Magn Reason Imaging 2021;53:1318–1343.} [1 – 2].
Introduction
Brain plasticity, the remarkable ability of the brain to reorganize and adapt, is most potent during sensitive periods in early childhood. These critical windows allow for significant neural development and learning, driven by environmental stimuli. However, this process also relies heavily on proper nutrition, which provides the essential building blocks for synaptic formation and overall brain health. Adequate nutrients such as glucose, oxygen, vitamins, iron, iodine, and fatty acids are crucial for maintaining brain plasticity and supporting cognitive functions. Early intervention during these sensitive periods is vital, as it leverages peak plasticity to address developmental delays and cognitive deficits effectively. The benefits of early intervention include improved cognitive abilities, better academic performance, and enhanced social skills, highlighting the importance of a holistic approach to brain development. Integrating timely environmental interactions and balanced nutrition optimizes learning and cognitive growth, setting a strong foundation for lifelong brain health.
Nutrition's Role in Brain Development: The Crucial Ingredient for Learning
It is clear that learning induces significant biological changes – not only do neurons modify their dendrites and axons, but the surrounding glial cells also undergo transformation. These changes take time, requiring a cascade of biological activities over several days. Many genes specializing in plasticity must be expressed to produce the necessary proteins and membranes for new synapses, dendrites, and axons. This process is energy-intensive, with a young child’s brain consuming up to 50% of the body’s energy. Essential nutrients like glucose, oxygen, vitamins, iron, iodine, and fatty acids are crucial for brain growth. The brain relies not just on intellectual stimulation but also on a balanced diet, oxygenation, and physical exercise [3].
A tragic incident in Israel in 2003 highlighted the brain’s sensitivity to nutrition [4]. Dozens of babies developed severe neurological symptoms after being fed soy-based milk powder devoid of thiamine (vitamin B1), a crucial nutrient. The manufacturer had omitted thiamine for economic reasons, leading to serious deficiencies. Thiamine deficiency in adults causes Wernicke-Korsakoff syndrome, marked by mental confusion, eye movement disorders, and even coma. The babies displayed similar symptoms, and their conditions improved only after thiamine was reintroduced. However, years later, many exhibited significant language impairments despite appearing normal in other cognitive aspects. This case illustrates the limitations of brain plasticity; while infants’ brains are highly plastic and capable of learning any language, this plasticity requires proper nutritional support. Even short-term deprivation can lead to lasting deficits, often confined to specific cognitive domains like grammar or vocabulary. Similar issues arise from fetal alcohol syndrome, caused by prenatal alcohol exposure, which severely impacts brain development. Overall, for optimal brain function and development, ensuring proper nutrition is essential [5].
Sensitive Periods: The Critical Window for Brain Plasticity and Learning
A sensitive period is a crucial timeframe in early brain development when plasticity is at its peak, allowing for significant changes in neural connections. Brain plasticity, while extensive, has its limits. Although neurons’ connections can adapt and evolve as we live, learn, and mature, the primary structures are present from birth and remain fundamentally consistent (see Figure 1). Learning involves minor adjustments within microcircuits, often spanning mere millimeters. As neurons mature, they develop new synaptic connections but remain within a fixed genetic framework. Environmental influences can alter local connectivity, synaptic strength, and myelination, which accelerates information transmission. However, neurons cannot reorient long-distance connections freely.
This inherent limitation is paired with a temporal constraint – brain plasticity peaks during early childhood, gradually waning with age. Sensory areas reach their highest plasticity around ages one to two, while higher-order regions like the prefrontal cortex peak later, during late childhood or early adolescence. With aging, plasticity decreases, making learning increasingly challenging.
Infants, often described as “learning machines,” exhibit remarkable synaptic plasticity in their early years. At birth, their cortex resembles a sparsely populated forest, which rapidly transforms into a dense jungle of neuronal connections within the first six months (see Figure 2). Although this growth suggests environmental influences, it is more complex. Synapses are overproduced initially, with the environment determining which are retained or pruned. Early childhood synaptic density is twice that of adults, decreasing over time. Each cortical region undergoes cycles of synaptic overproduction and selective pruning, based on utility [6-8]. (Cf. previous blogs entitled as: Brain Plasticity – I: Synapses – The Mushrooms of Learning; Brain Plasticity – II: But the Butterflies of the Brain – Neuronal Elegance Unveiled).
This process explains the concept of sensitive periods, where the dendritic and synaptic structures are highly adaptable in early childhood. As the brain matures, learning becomes confined to more minor changes. This dynamic process underscores the critical importance of early experiences in shaping neural development and lifelong learning capabilities.
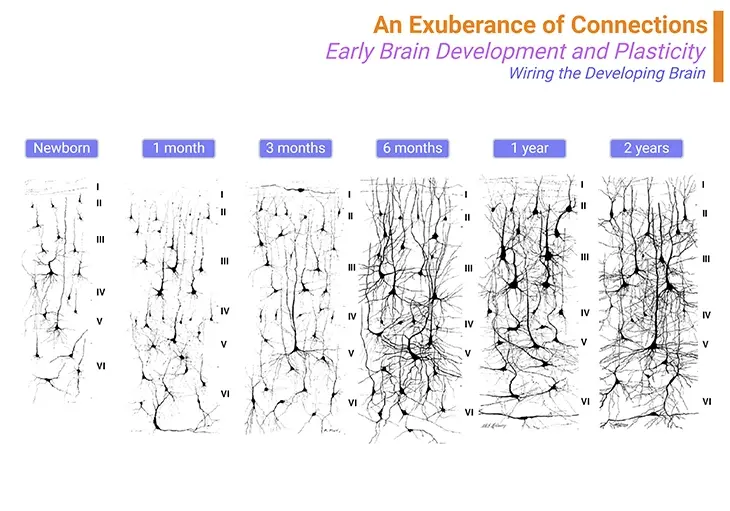
Figure 2. An exuberance of connections ~ Wiring the developing brain. The child’s cortex is a jungle of connections. At birth, most cortical neurons are thin and sparse. However, within the first six months of life, dendritic and axonal branches multiply rapidly, forming a dense thicket. Paradoxically, synapses are more numerous in children aged one to two than in adults. This overproduction allows for selective learning. Effective microcircuits are preserved and amplified, while ineffective ones are pruned. Pruning happens at different rates across the cortex. Sensory areas mature first, with synaptic overproduction peaking around two years old and stabilizing after a few years. This is why early intervention for issues like amblyopia or strabismus is critical. The prefrontal cortex peaks much later, with synaptic elimination continuing until the mid-20s. Our educational system aligns with this biological timeline, training children until their plasticity declines. In adults, individual synapses may remain stable for long periods. {Image credit: Postmortem images obtained by Jesse LeRoy Conel in the mid-twentieth century at Harvard University.}.
These waves of synaptic overproduction and pruning do not happen uniformly across the brain. Sensory regions like the primary visual cortex mature faster than higher-level cortical areas. The principle seems to be to stabilize the brain’s inputs early in sensory areas while keeping higher-level areas more flexible for a longer time. Consequently, higher cortical areas like the prefrontal cortex are the last to stabilize, continuing to change through adolescence and beyond. In humans, synaptic overproduction peaks around two years in the visual cortex, three to four years in the auditory cortex, and five to ten years in the prefrontal cortex. Myelination, which insulates axons to speed up information transmission, follows a similar pattern. Early in life, sensory neurons benefit first from myelination, drastically speeding up visual processing. However, this process is slower in the frontal cortex, responsible for abstract thought, attention, and planning. Thus, young children have ‘mature sensory and motor circuits but slower high-level cognitive circuits.’
Sensitive periods for learning open and close at different times depending on the brain region. Early sensory areas lose their learning ability first. For example, binocular vision, the ability to merge visual information from both eyes to perceive depth, develops only if the visual cortex gets high-quality input from both eyes during a sensitive period. If one eye is impaired during this time, the brain’s ability to merge the images can be permanently lost, resulting in amblyopia (“lazy eye”). This condition must be corrected early in life, ideally before age three. Another sensitive period involves language acquisition. Babies can distinguish all phonemes at birth, but their ability to do so diminishes unless they are exposed to language. For instance, Japanese speakers may struggle to distinguish the sounds /R/ and /L/ later in life, highlighting the critical nature of early language exposure.
Research indicates that our ability to learn phonemes diminishes by the end of the first year of life. As infants, we unconsciously analyze the sounds around us, and our brains adjust to the phonemes used in our environment. By around twelve months, this process stabilizes, and our capacity to learn new phonemes decreases significantly. Consequently, as adults, we find it challenging to achieve native-like pronunciation in languages such as Finnish or Hindi. Recovering the ability to distinguish foreign phonemes requires intense and focused rehabilitation, gradually training our ears to hear and differentiate sounds.
This phenomenon is why scientists refer to a “sensitive period” rather than a “critical period” – while the capacity for learning shrinks, it never entirely disappears. In adulthood, the ability to learn new phonemes varies significantly among individuals, making foreign language acquisition a daunting task for most. The sensitive period for mastering phonology ends early in life, whereas higher-level language skills like grammar remain malleable until around puberty.
Early childhood is crucial for the development of syntax. Without linguistic interactions by the end of the first year, brain plasticity for syntax declines. For example, thiamine deprivation in Israeli infants in 2003 led to a permanent loss of syntactic abilities. Language learning exemplifies sensitive periods, with distinct phases for phonology and grammar. However, the brain’s capacity to learn new vocabulary remains throughout life, allowing adults to acquire new words and their meanings, albeit with varying ease. This residual plasticity enables us to adapt to new linguistic concepts, although the exact biological mechanisms behind this phenomenon remain unknown.
Take Home Messages
Sensitive Periods of Learning
- Neural Plasticity: Brain plasticity peaks during sensitive periods in early childhood, allowing for significant synaptic growth and pruning.
- Temporal Dynamics: Different brain regions have varying timelines for peak plasticity, with sensory areas maturing earlier and higher cognitive regions maturing later.
- Learning Constraints: While plasticity decreases with age, learning remains possible, albeit more challenging.
Nutrition’s Role in Brain Development
- Critical Nutrition: Proper nutrition is vital for brain development, influencing synaptic formation, dendritic growth, and overall neural health.
- Energy Demands: A young child’s brain consumes up to 50% of the body’s energy, underscoring the need for a balanced diet rich in essential nutrients.
- Impact of Deficiency: Nutritional deficiencies can lead to severe and lasting cognitive impairments, as illustrated by the thiamine deficiency incident in Israel.
Combined Insights
- Integrated Approach: Optimal learning and brain development require a combination of timely environmental stimulation and adequate nutritional support.
- Long-Term Effects: Early childhood experiences and nutrition have lasting impacts on cognitive and linguistic abilities, highlighting the importance of addressing both during critical development periods.
These key points underscore the intricate interplay between brain plasticity and nutrition in shaping cognitive development and lifelong learning capabilities.
Summary and Conclusions
Brain plasticity is a fundamental aspect of cognitive development, particularly during sensitive periods in early childhood when synaptic growth and pruning are most dynamic. Optimal brain function and learning require a combination of timely environmental stimulation and adequate nutrition. Proper nutrition is vital, as deficiencies can lead to lasting cognitive impairments. As we age, neural plasticity declines, making early experiences and nutrition crucial for long-term cognitive abilities. Together, these insights underscore the importance of a holistic approach to fostering brain health and learning.
For information on autism monitoring, screening and testing please read our blog.
References
- Dubois J, Alison M, Counsell SJ, Hertz-Pannier L, Hüppi PS, Benders MJNL. MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. J Magn Reson Imaging. 2021 May;53(5):1318-1343. doi: 10.1002/jmri.27192. Epub 2020 May 18. PMID: 32420684; PMCID: PMC8247362.
https://pubmed.ncbi.nlm.nih.gov/32420684/ - Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014 Sep 12;276:48-71. doi: 10.1016/j.neuroscience.2013.12.044. Epub 2013 Dec 28. PMID: 24378955.
https://pubmed.ncbi.nlm.nih.gov/24378955/ - Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014 Apr;72(4):267-84. doi: 10.1111/nure.12102. Epub 2014 Mar 28. PMID: 24684384.
https://pubmed.ncbi.nlm.nih.gov/24684384/ - Shamir R. Thiamine-deficient infant formula: what happened and what have we learned? Ann Nutr Metab. 2012;60(3):185-7. doi: 10.1159/000338211. Epub 2012 Jun 6. PMID: 22699764.
https://pubmed.ncbi.nlm.nih.gov/22699764/ - Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015 May;27(2):411-23. doi: 10.1017/S0954579415000061. PMID: 25997762; PMCID: PMC4443711.
https://pubmed.ncbi.nlm.nih.gov/25997762/ - Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004 Oct;16(8):1412-25. doi: 10.1162/0898929042304796. PMID: 15509387.
https://pubmed.ncbi.nlm.nih.gov/15509387/
- Kolb B, Harker A, Gibb R. Principles of plasticity in the developing brain. Dev Med Child Neurol. 2017 Dec;59(12):1218-1223. doi: 10.1111/dmcn.13546. Epub 2017 Sep 13. PMID: 28901550.
https://pubmed.ncbi.nlm.nih.gov/28901550/
- Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012 Jun 20;13(7):478-90. doi: 10.1038/nrn3258. PMID: 22714019.
https://pubmed.ncbi.nlm.nih.gov/22714019/